- Mix With Cold Water, Sodium Carbonate Dissolves
- Filter off Lead (II) Chloride And Calcium sulphate as residue
- Evaporate to obtain sodium carbonate
- Mix the residue with hot water to dissolve Lead (II) chloride
- Filter off Calcium sulphate as a residue dry over dessicator
- Cool the filtrate to precipitate Lead (II) chloride
- Filter off residue as Lead (II chloride and dry
sharon kalunda answered the question on May 17, 2019 at 05:36
-
In an experiment,1g of calcium carbonate was completely dissolved 100cm3 of 0.25M excess hydrochloric acid.Calculate the molar concentration of the acidic solution formed. (Ca = 40;...
(Solved)
In an experiment,1g of calcium carbonate was completely dissolved 100cm3 of 0.25M excess hydrochloric acid.Calculate the molar concentration of the acidic solution formed. (Ca = 40; C = 12; O =16).
Date posted:
May 17, 2019
.
Answers (1)
-
Explain the meanings of the following physical properties of laboratory gases.
(Solved)
Explain the meanings of the following physical properties of laboratory gases.
i) A chocking smell.
ii) An irritating smell.
iii) A neutral gas
Date posted:
May 16, 2019
.
Answers (1)
-
An element X has atomic number 3, relative atomic mass 6.94 and consists of two isotopes
of mass numbers 6 and 7.
(Solved)
An element X has atomic number 3, relative atomic mass 6.94 and consists of two isotopes
of mass numbers 6 and 7.
a) What is the mass number of the more abundant isotope of X?
b) Calculate the relative abundance of each of the isotopes.
Date posted:
May 16, 2019
.
Answers (1)
-
State two large scale uses of hard water.
(Solved)
State two large scale uses of hard water.
Date posted:
May 16, 2019
.
Answers (1)
-
Explain how dilute hydrochloric acid can be used to determine the type of hardness in a sample of tap water.
(Solved)
Explain how dilute hydrochloric acid can be used to determine the type of hardness in a sample of tap water.
Date posted:
May 16, 2019
.
Answers (1)
-
A factory produces 63.6 tonnes of anhydrous Na2CO3 on a certain. Calculate the number of tonnes of sodium chloride used on this particular day. Assume...
(Solved)
A factory produces 63.6 tonnes of anhydrous Na2CO3 on a certain. Calculate the number of tonnes of sodium chloride used on this particular day. Assume the plant is working at 100% efficiency.(C = 12, H = 1, Cl = 35.5, Ca = 40, Na = 23)
Date posted:
May 16, 2019
.
Answers (1)
-
The flow chart below shows the manufacture of sodium carbonate. Study it carefully and
answer the questions that follow.
(Solved)
The flow chart below shows the manufacture of sodium carbonate. Study it carefully and
answer the questions that follow.
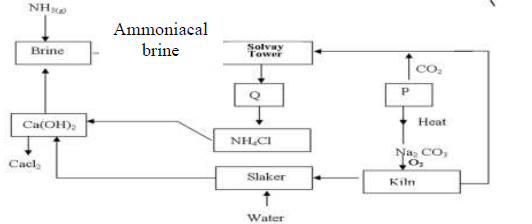
a)
i). What is ammoniacal brine?
ii). Ammoniacal brine reacts with carbon (IV) oxide to form a mixture of two salts
which produce Q. Write an equation to show formation of Q
Name two processes that are used to separate Q into NH4Cl and P
b) Give two uses of sodium carbonate produced in the process.
c)i). Name the substance that reacts with water that comes into the slaker
ii). What happens at the kiln?
d) Write an equation for the reaction that occurs when P is heated to form solid
Na2 CO3
e) Name two substances that are recycled in the process.
Date posted:
May 16, 2019
.
Answers (1)
-
Define the term molar heat of displacement
(Solved)
Define the term molar heat of displacement
Date posted:
May 16, 2019
.
Answers (1)
-
0.65 of zinc was reacted with 20cm3 of 2 M copper(II) sulphate solution in a plastic beaker.
The copper(II) sulphate solution was in excess. The initial...
(Solved)
0.65 of zinc was reacted with 20cm3 of 2 M copper(II) sulphate solution in a plastic beaker.
The copper(II) sulphate solution was in excess. The initial temperature and the highest temperature of the solution were recorded. 0.64 of copper metal was formed.
a) Other than change in temperature, state the observations made during the reaction
b) Calculate the
I Number of moles of Zinc that reacted (Zn=65)
II The number of moles of copper that was displaced from the solution (Cu=64)
III The mole ratio of Zn: Cu
Date posted:
May 16, 2019
.
Answers (1)
-
The table below gives the percentage of a radioactive isotope of Bismuth that remains after decaying at different times.
(Solved)
The table below gives the percentage of a radioactive isotope of Bismuth that remains after
decaying at different times.

i). On the grid provided, plot a graph of the percentage of Bismuth remaining(Vertical axis) against
time.
ii) Using the graph determine the
i) Half- life of Bismuth isotope.
ii) Original mass of the Bismuth Isotope given that the mass that remained after 70
minutes was 0.16g.
Date posted:
May 16, 2019
.
Answers (1)
-
Study the flow chart below and use it to answer the questions between.
(Solved)
Study the flow chart below and use it to answer the questions between.

a) Identify the process described by the flow chart
b) Explain why the Ore is crushed
c) Which process occurs at mixing chamber?
d) Explain the use of ;
i) water
ii) Oil
e) Compressed air Write down an equation for the formation of slag.
f) Identify the cations present where the metal is being purified.
Date posted:
May 16, 2019
.
Answers (1)
-
The set – up below was used during the electrolysis of a solution of magnesium
sulphate using inert electrodes.
(Solved)
The set – up below was used during the electrolysis of a solution of magnesium
sulphate using inert electrodes.

i). Identify the ions present in the electrolyte
ii). Write half equations at the anode and at the cathode:
Cathode:
Anode
Date posted:
May 16, 2019
.
Answers (1)
-
Dry chlorine gas was passed through two pieces of coloured cotton cloth as shown.
(Solved)
Dry chlorine gas was passed through two pieces of coloured cotton cloth as shown.

a) State what is observed in each experiment.
Experiment 1
Experiment 2
b) Explain your observation using an equation.
Date posted:
May 16, 2019
.
Answers (1)
-
During the extraction of copper and Zinc from their Ores, some of the processes include.
(i) Crushing
(ii) Mixing of the crushed Ore with Oil and water...
(Solved)
During the extraction of copper and Zinc from their Ores, some of the processes include.
(i) Crushing
(ii) Mixing of the crushed Ore with Oil and water and bubbling air through it.
(i) Name the process(ii) above.
(ii) What is the purpose of process (ii) above?
Date posted:
May 16, 2019
.
Answers (1)
-
The boiling and melting points of alkali metals decreases down the group while the melting and boiling points of halogens increase down the group.Explain
(Solved)
The boiling and melting points of alkali metals decreases down the group while the melting and boiling points of halogens increase down the group.Explain
Date posted:
May 16, 2019
.
Answers (1)
-
Helium is used instead of Hydrogen to fill balloons.Explain
(Solved)
Helium is used instead of Hydrogen to fill balloons.Explain
Date posted:
May 16, 2019
.
Answers (1)
-
A solution containing 0.1m sulphuric acid has a pH of 2 while 5M has a pH of more than two.
Explain.
(Solved)
A solution containing 0.1m sulphuric acid has a pH of 2 while 5M has a pH of more than two.
Explain.
Date posted:
May 16, 2019
.
Answers (1)
-
State and explain the observation made in the set-up below.
(Solved)
State and explain the observation made in the set-up below.

Date posted:
May 16, 2019
.
Answers (1)
-
A mixture of carbon (IV) Oxide and carbon (II) oxide is passed through potassium hydroxide
solution as shown in the following set up.
(Solved)
A mixture of carbon (IV) Oxide and carbon (II) oxide is passed through potassium hydroxide
solution as shown in the following set up.

I) Name gas X
(ii) Why should gas X be burned.
(iii) Write a well balanced chemical equation for the reaction that takes place in the conical
flask in the first few seconds.
Date posted:
May 16, 2019
.
Answers (1)
-
Write ionic equations for electrolysis of dilute sulphuric acid using platinum electrodes at:
(Solved)
Write ionic equations for electrolysis of dilute sulphuric acid using platinum electrodes at:
(i) Anode
(ii) Cathode
Date posted:
May 16, 2019
.
Answers (1)