- Write a chemical equation to represent the chemical reaction between an acid and
water.(Solved)
Write a chemical equation to represent the chemical reaction between an acid and
water.
Date posted: May 17, 2019. Answers (1)
- Name the polymer with the following structural formula and state its commercial use.(Solved)
Name the polymer with the following structural formula and state its commercial use.
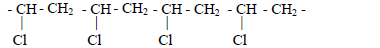
Date posted: May 17, 2019. Answers (1)
- Describe how you would obtain pure solid samples of each of the following components of a
solid mixture containing ; Lead (II) chloride, Sodium carbonate and...(Solved)
Describe how you would obtain pure solid samples of each of the following components of a
solid mixture containing ; Lead (II) chloride, Sodium carbonate and calcium sulphate.
Date posted: May 17, 2019. Answers (1)
- In an experiment,1g of calcium carbonate was completely dissolved 100cm3 of 0.25M excess hydrochloric acid.Calculate the molar concentration of the acidic solution formed. (Ca = 40;...(Solved)
In an experiment,1g of calcium carbonate was completely dissolved 100cm3 of 0.25M excess hydrochloric acid.Calculate the molar concentration of the acidic solution formed. (Ca = 40; C = 12; O =16).
Date posted: May 17, 2019. Answers (1)
- A hydrocarbon has an empirical formula C2H3and a relative molecular mass of 54.(Solved)
A hydrocarbon has an empirical formula C2H3and a relative molecular mass of 54.
a) Determine the molecular formula of the hydrocarbon ( C=12; H=1)
b) Name the homologous series to which the hydrocarbon belongs. Give a reason for your answer.
c) When one mole of the hydrocarbon reacts with one mole of hydrogen chloride gas,
compound W is formed. Give the IUPAC systematic name of W.
Date posted: May 16, 2019. Answers (1)
- The following grid represents an extract of a periodic table. Use the grid to answer the
questions that follow.(Solved)
The following grid represents an extract of a periodic table. Use the grid to answer the
questions that follow.
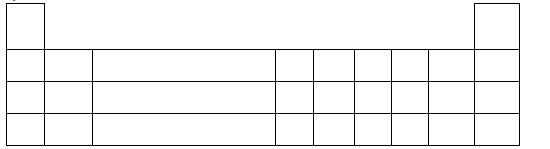
On the grid above;
a) Indicate by means of an arrow showing an increasing trend in the reducing power
of elements.
b) Mark element J a metal and element Q a non-metal, such that compound J,Q, has
the highest ionic character. Explain.
Date posted: May 16, 2019. Answers (1)
- Explain the meanings of the following physical properties of laboratory gases.(Solved)
Explain the meanings of the following physical properties of laboratory gases.
i) A chocking smell.
ii) An irritating smell.
iii) A neutral gas
Date posted: May 16, 2019. Answers (1)
- An element X has atomic number 3, relative atomic mass 6.94 and consists of two isotopes
of mass numbers 6 and 7.(Solved)
An element X has atomic number 3, relative atomic mass 6.94 and consists of two isotopes
of mass numbers 6 and 7.
a) What is the mass number of the more abundant isotope of X?
b) Calculate the relative abundance of each of the isotopes.
Date posted: May 16, 2019. Answers (1)
- When burning magnesium ribbon is put into a gas jar of carbon (IV) oxide gas, it continues to
burn leaving behind white solid powder and black...(Solved)
When burning magnesium ribbon is put into a gas jar of carbon (IV) oxide gas, it continues to
burn leaving behind white solid powder and black solid specks as residue write chemical
equation for the reaction that produces.
i) The white solid powder.
ii) Black solid specks.
Date posted: May 16, 2019. Answers (1)
- State two large scale uses of hard water.(Solved)
State two large scale uses of hard water.
Date posted: May 16, 2019. Answers (1)
- Explain how dilute hydrochloric acid can be used to determine the type of hardness in a sample of tap water.(Solved)
Explain how dilute hydrochloric acid can be used to determine the type of hardness in a sample of tap water.
Date posted: May 16, 2019. Answers (1)
- Describe hardness of water.(Solved)
Describe hardness of water.
Date posted: May 16, 2019. Answers (1)
- A factory produces 63.6 tonnes of anhydrous Na2CO3 on a certain. Calculate the number of tonnes of sodium chloride used on this particular day. Assume...(Solved)
A factory produces 63.6 tonnes of anhydrous Na2CO3 on a certain. Calculate the number of tonnes of sodium chloride used on this particular day. Assume the plant is working at 100% efficiency.(C = 12, H = 1, Cl = 35.5, Ca = 40, Na = 23)
Date posted: May 16, 2019. Answers (1)
- The flow chart below shows the manufacture of sodium carbonate. Study it carefully and
answer the questions that follow.(Solved)
The flow chart below shows the manufacture of sodium carbonate. Study it carefully and
answer the questions that follow.
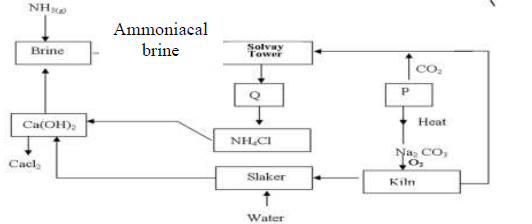
a)
i). What is ammoniacal brine?
ii). Ammoniacal brine reacts with carbon (IV) oxide to form a mixture of two salts
which produce Q. Write an equation to show formation of Q
Name two processes that are used to separate Q into NH4Cl and P
b) Give two uses of sodium carbonate produced in the process.
c)i). Name the substance that reacts with water that comes into the slaker
ii). What happens at the kiln?
d) Write an equation for the reaction that occurs when P is heated to form solid
Na2 CO3
e) Name two substances that are recycled in the process.
Date posted: May 16, 2019. Answers (1)
- Define the term molar heat of displacement(Solved)
Define the term molar heat of displacement
Date posted: May 16, 2019. Answers (1)
- 0.65 of zinc was reacted with 20cm3 of 2 M copper(II) sulphate solution in a plastic beaker.
The copper(II) sulphate solution was in excess. The initial...(Solved)
0.65 of zinc was reacted with 20cm3 of 2 M copper(II) sulphate solution in a plastic beaker.
The copper(II) sulphate solution was in excess. The initial temperature and the highest temperature of the solution were recorded. 0.64 of copper metal was formed.
a) Other than change in temperature, state the observations made during the reaction
b) Calculate the
I Number of moles of Zinc that reacted (Zn=65)
II The number of moles of copper that was displaced from the solution (Cu=64)
III The mole ratio of Zn: Cu
Date posted: May 16, 2019. Answers (1)
- The grid below represents part of the periodic table. The letters do not represent the actual
symbols of the elements. Study it and answer the questions...(Solved)
The grid below represents part of the periodic table. The letters do not represent the actual
symbols of the elements. Study it and answer the questions that follow.

a) Explain why element A is placed in two positions in the periodic table.
b) Name the type of bond and structure formed when element F reacts with element G.
c) Select one element which forms a highly soluble carbonate.
d) Which name is given to the group to which element D belong?.
e) Compare and explain the following:
i). Atomic radii of elements B and E.
ii). Electrical conductivity of elements E and H.
f)i). The oxide of element C was dissolved in water to form a solution of C. Compare the pH
value of the solution C with that of sodium chloride solution. Give a reason for your answer.
ii). Give ONE use of elements of which J is a member.
Date posted: May 16, 2019. Answers (1)
- State one use of radioactive isotope in (i). Medicine (ii). Industry.(Solved)
State one use of radioactive isotope in
i). Medicine
ii). Industry.
Date posted: May 16, 2019. Answers (1)
- Radioactive carbon – 14 decays by emitting ß- Particle to form N-14. Write a nuclear
equation for the reaction.(Solved)
Radioactive carbon – 14 decays by emitting β- Particle to form N-14. Write a nuclear
equation for the reaction.
Date posted: May 16, 2019. Answers (1)
- Distinguish between nuclear fission and nuclear fusion.(Solved)
Distinguish between nuclear fission and nuclear fusion.
Date posted: May 16, 2019. Answers (1)