- Aluminium ions are above hydrogen ions in the electrochemical series.
- Hydrogen gas evolved instead aluminium deposited.
- Copper II ions below H+ ions Cu2+ ions are discharged Copper is deposited at the cathode.
sharon kalunda answered the question on May 17, 2019 at 07:04
- Study the following equilibrium equation and answer the questions that follows.(Solved)
Study the following equilibrium equation and answer the questions that follows.
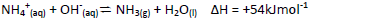
Explain how ammonia gas can be prepared in the laboratory.
Date posted: May 17, 2019. Answers (1)
- The following are standard electrode potentials of some half-cell reactions. Use the data to
answer the questions that follow.(Solved)
The following are standard electrode potentials of some half-cell reactions. Use the data to
answer the questions that follow.
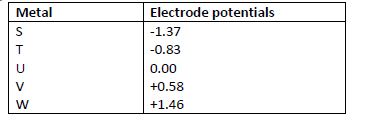
i) Suggest the identify of element U.
ii) Draw a labeled diagram of an electrochemical cell that would produce the largest e.m.f.
Date posted: May 17, 2019. Answers (1)
- The following diagram represents a set-up used to investigate conditions necessary for rusting of iron.(Solved)
The following diagram represents a set-up used to investigate conditions necessary for rusting of iron.
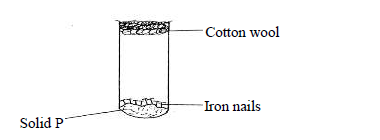
After several days it was found that the nails did not rust. Identify solid P.
Date posted: May 17, 2019. Answers (1)
- The following diagram was used to investigate the electrolysis of copper(II) Sulphate solution
and molten G chloride using carbon electrodes.(Solved)
The following diagram was used to investigate the electrolysis of copper(II) Sulphate solution
and molten G chloride using carbon electrodes.
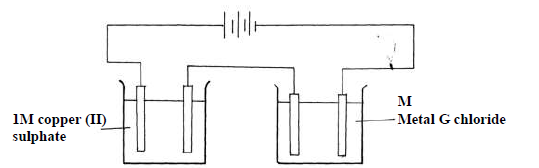
When a fixed current was passed through the two electrolytic cells as shown, 1.27g of copper
and 0.6g of G were deposited at the respective electrodes,. Calculate the numerical value
of x in the formula Gx+
(Cu=63.5; g = 60; 1 Faraday = 96500C)
Date posted: May 17, 2019. Answers (1)
- In the chemistry laboratory, both blue and red litmus papers are used to test for the nature
of gases and solutions. Explain(Solved)
In the chemistry laboratory, both blue and red litmus papers are used to test for the nature
of gases and solutions. Explain
Date posted: May 17, 2019. Answers (1)
- Explain why graphite is preferred to lubricating oil in the moving parts of the machine.(Solved)
Explain why graphite is preferred to lubricating oil in the moving parts of the machine.
Date posted: May 17, 2019. Answers (1)
- Q grams of a radioactive isotope sample takes 80 days to disintegrate to 7g. The halflife of the isotope is 20days. Find the initial mass...(Solved)
Q grams of a radioactive isotope sample takes 80 days to disintegrate to 7g. The halflife of the isotope is 20days. Find the initial mass Q.
Date posted: May 17, 2019. Answers (1)
- In the laboratory, chlorine gas can be prepared by the reaction;(Solved)
In the laboratory, chlorine gas can be prepared by the reaction;

Given the following half- cell reactions;
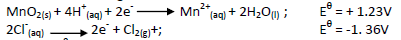
Use the Eθ cell to explain how the above reaction is carried out in the laboratory.
Date posted: May 17, 2019. Answers (1)
- State the main differences between alkanes and alkenes in terms of the following;(Solved)
State the main differences between alkanes and alkenes in terms of the following;
i) Structure and bonding
ii) Reaction with chlorine gas.
Date posted: May 17, 2019. Answers (1)
- A fixed mass of a gas has a volume of 250cm3 at 27oC and 750mmHg pressure.
Calculate thegas volume that the gas would occupy at 41oC...(Solved)
A fixed mass of a gas has a volume of 250cm3 at 27oC and 750mmHg pressure.
Calculate thegas volume that the gas would occupy at 41oC and 750mmHg pressure.
(0o = 273k)
Date posted: May 17, 2019. Answers (1)
- When chlorine gas is dissolved in water it acts as a bleaching agent.(Solved)
When chlorine gas is dissolved in water it acts as a bleaching agent.
a) Write a chemical equation to show the role of water in the bleaching property of chlorine.
b) Name the chlorine compound that is present in the commercial bleaching agents.Give a reason for your answer.
Date posted: May 17, 2019. Answers (1)
- The heat of combustion of carbon, hydrogen and methane are 405kJ/mol, 286kJ/mol and886kJ/mol respectively.(Solved)
The heat of combustion of carbon, hydrogen and methane are 405kJ/mol, 286kJ/mol and886kJ/mol respectively.
Calculate the heat change for the reaction, ΔH.
C(s) + 2H2(g) ------> CH4(g); ΔH
Date posted: May 17, 2019. Answers (1)
- When aqueous potassium hydroxide is electrolysed using platinum electrodes, hydrogen gas
is produced at the cathode.(Solved)
When aqueous potassium hydroxide is electrolysed using platinum electrodes, hydrogen gas
is produced at the cathode.
a) Give a reason why platinum is described as an inert electrode.
b) Explain how hydrogen gas is produced in this experiment.
Date posted: May 17, 2019. Answers (1)
- Write a chemical equation to represent the chemical reaction between an acid and
water.(Solved)
Write a chemical equation to represent the chemical reaction between an acid and
water.
Date posted: May 17, 2019. Answers (1)
- Name the polymer with the following structural formula and state its commercial use.(Solved)
Name the polymer with the following structural formula and state its commercial use.
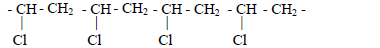
Date posted: May 17, 2019. Answers (1)
- Describe how you would obtain pure solid samples of each of the following components of a
solid mixture containing ; Lead (II) chloride, Sodium carbonate and...(Solved)
Describe how you would obtain pure solid samples of each of the following components of a
solid mixture containing ; Lead (II) chloride, Sodium carbonate and calcium sulphate.
Date posted: May 17, 2019. Answers (1)
- In an experiment,1g of calcium carbonate was completely dissolved 100cm3 of 0.25M excess hydrochloric acid.Calculate the molar concentration of the acidic solution formed. (Ca = 40;...(Solved)
In an experiment,1g of calcium carbonate was completely dissolved 100cm3 of 0.25M excess hydrochloric acid.Calculate the molar concentration of the acidic solution formed. (Ca = 40; C = 12; O =16).
Date posted: May 17, 2019. Answers (1)
- A hydrocarbon has an empirical formula C2H3and a relative molecular mass of 54.(Solved)
A hydrocarbon has an empirical formula C2H3and a relative molecular mass of 54.
a) Determine the molecular formula of the hydrocarbon ( C=12; H=1)
b) Name the homologous series to which the hydrocarbon belongs. Give a reason for your answer.
c) When one mole of the hydrocarbon reacts with one mole of hydrogen chloride gas,
compound W is formed. Give the IUPAC systematic name of W.
Date posted: May 16, 2019. Answers (1)
- The following grid represents an extract of a periodic table. Use the grid to answer the
questions that follow.(Solved)
The following grid represents an extract of a periodic table. Use the grid to answer the
questions that follow.
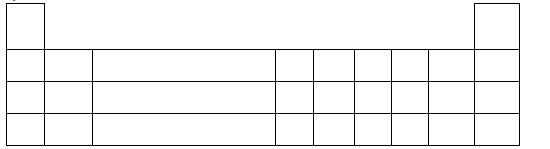
On the grid above;
a) Indicate by means of an arrow showing an increasing trend in the reducing power
of elements.
b) Mark element J a metal and element Q a non-metal, such that compound J,Q, has
the highest ionic character. Explain.
Date posted: May 16, 2019. Answers (1)
- Explain the meanings of the following physical properties of laboratory gases.(Solved)
Explain the meanings of the following physical properties of laboratory gases.
i) A chocking smell.
ii) An irritating smell.
iii) A neutral gas
Date posted: May 16, 2019. Answers (1)