- When a current of 2.0 amperes was passed through a cell containing aqueous solution of T2+
ions of metal T for 9 minutes the mass of...(Solved)
When a current of 2.0 amperes was passed through a cell containing aqueous solution of T2+
ions of metal T for 9 minutes the mass of the cathode increased by 0.36g.(1Faraday=96,500coulombs)
(a) Calculate the quantity of electricity used.
(b) Determine the relative atomic mass of metal T.
(c) Explain whether metal T is less or more reactive than hydrogen gas.
Date posted: May 17, 2019. Answers (1)
- In an experiment 1.2g of granulated zinc were reacted with excess 2.0M sulphuric acid. The
time taken for the reaction to be completed was recorded. The...(Solved)
In an experiment 1.2g of granulated zinc were reacted with excess 2.0M sulphuric acid. The
time taken for the reaction to be completed was recorded. The experiment was repeated using
1.2g of zinc powder.
(a) In which experiment was the time taken shorter? Explain your answer.
(b) The mass of remaining mass of zinc was measured as time moved until when the
reaction was over. Sketch on the set of axes and label the two curves obtained that
would represent the mass of the remaining zinc
Experiment I represents granulated zinc.
Experiment II represents zinc powder.

Date posted: May 17, 2019. Answers (1)
- The set up below was used by a student. Filter paper soaked in purple litmus solution was
placed in the middle of the combustion tube.(Solved)
The set up below was used by a student. Filter paper soaked in purple litmus solution was
placed in the middle of the combustion tube.

(i) What is the main aim of the experiment.
(ii) State the first observation likely to have been made in the tube. Explain the observation.
Date posted: May 17, 2019. Answers (1)
- 0.5g of Manganese (IV) oxide were added to 50 cm3 of 3.5M hydrogen peroxide. The
temperature of the solution rose from 21oC to 64oC. The information...(Solved)
0.5g of Manganese (IV) oxide were added to 50 cm3 of 3.5M hydrogen peroxide. The
temperature of the solution rose from 21oC to 64oC. The information was represented on an energy level diagram as shown.
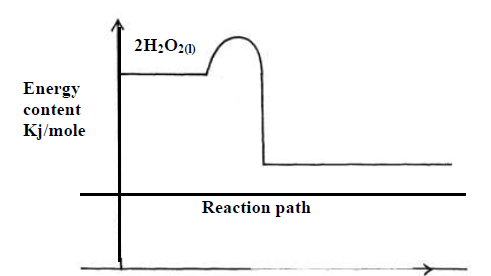
(a) Determine the number of moles of hydrogen peroxide that decomposed.
(b) Calculate the molar enthalpy of decomposition of hydrogen peroxide.
(c) On the same set of axes above sketch the curve that would be obtained if manganese (IV)
oxide was not used and other conditions remained constant.
Date posted: May 17, 2019. Answers (1)
- In a reaction, an alkanol B was converted to pent-2-ene(Solved)
In a reaction, an alkanol B was converted to pent-2-ene
(a) Give the structural formula of alkanol B.
(b) Name (i) the type of reaction that converts alkanol B to pent-2-ene.
(ii) The reagent used.
Date posted: May 17, 2019. Answers (1)
- The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that...(Solved)
The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that follow.
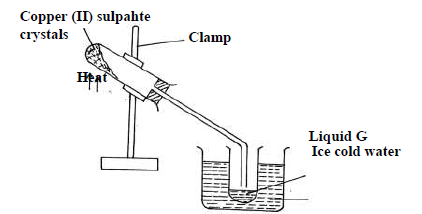
(a) Why is boiling tube slanted as shown?
(b) What is observed in the boiling tube.
(c) Identify liquid G.
Date posted: May 17, 2019. Answers (1)
- Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.(Solved)
Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.
(i) Zinc + dilute nitric acid.
(ii) Lead + dilute hydrochloric acid.
(iii) Potassium + dilute sulphuric acid.
Date posted: May 17, 2019. Answers (1)
- Give names of the following processes used to:
(Solved)
Give names of the following processes used to:
(a) Obtain a solvent from a saturated solution.
(b) Remove steam from air
(c) Separate zinc carbonate from water
(d) Separate a mixture of nitrogen and helium.
Date posted: May 17, 2019. Answers (1)
- Describe a simple test that can be carried out in the laboratory to distinguish between manganese (IV) oxide and copper (II) oxide.(Solved)
Describe a simple test that can be carried out in the laboratory to distinguish between manganese (IV) oxide and copper (II) oxide.
Date posted: May 17, 2019. Answers (1)
- State three properties common to both ammonia and calcium hydroxide solutions but different
from solution of sulphur (IV) oxide in water.(Solved)
State three properties common to both ammonia and calcium hydroxide solutions but different
from solution of sulphur (IV) oxide in water.
Date posted: May 17, 2019. Answers (1)
- A current of 25 amps was passed through molten aluminium oxide for 36 hrs.Calculate the amount of aluminium deposited in kg.(Al = 27, IF =...(Solved)
A current of 25 amps was passed through molten aluminium oxide for 36 hrs.Calculate the amount of aluminium deposited in kg.(Al = 27, IF = 96500C)
Date posted: May 17, 2019. Answers (1)
- Aluminium is extracted from its ore by electrolysis method. The current required in the
process is 4,000 amperes. Study the diagram and answer the questions that...(Solved)
Aluminium is extracted from its ore by electrolysis method. The current required in the
process is 4,000 amperes. Study the diagram and answer the questions that follow.
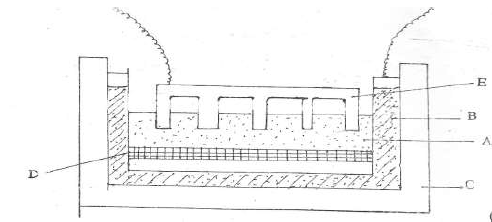
(a) Name:
(i) Electrolyte A
(ii) Substance D
(iii) Electrode E
(b) Name the material from which the electrodes are made.
(c ) (i) Write the equation that produces aluminium metal.
(ii) Explain why E has to be replaced from time to time.
(iii) Why is cryolite added to the electrolyte before the process of electrolysis ?
Date posted: May 17, 2019. Answers (1)
- In an experiment to determine the molar heat of neutralization of hydrochloric acid
with sodium hydroride, students of Furaha Secondary school reacted 100cm3 of 1M
hydrochloric acid...(Solved)
In an experiment to determine the molar heat of neutralization of hydrochloric acid
with sodium hydroride, students of Furaha Secondary school reacted 100cm3 of 1M
hydrochloric acid with 50cm3 of 2M sodium hydroxide solution. They obtained the following results.
Initial temperature of acid = 25.00C
Initial temperature of base = 25.00C
Highest temperature reached
With the acid – alkali mixture = 34.00C
(a) Write an ionic equation for the neutralization reaction between hydrochloric acid
and sodium hydroxide.
(b ) Calculate :
(i) The change in temperature.
(ii) The amount of heat produced during the reaction.
( Specific heat capacity of solution = 4.2 kJkg-1k-1 )
(iii) The molar heat of neutralization of sodium hydroxide.
(c) Write the thermochemical equation for the reaction.
(d) Draw an energy level diagram for the reaction.
Date posted: May 17, 2019. Answers (1)
- Define the term molar heat of neutralization.(Solved)
Define the term molar heat of neutralization.
Date posted: May 17, 2019. Answers (1)
- State two uses of carbon (IV) oxide.(Solved)
State two uses of carbon (IV) oxide.
Date posted: May 17, 2019. Answers (1)
- State one environmental effect that excess carbon (IV) oxide in the air causes.(Solved)
State one environmental effect that excess carbon (IV) oxide in the air causes.
Date posted: May 17, 2019. Answers (1)
- A piece of marble chip ( calcium carbonate) is put in a beaker containing excess of dilute hydrochloric
acid which is placed on a reading balance....(Solved)
A piece of marble chip ( calcium carbonate) is put in a beaker containing excess of dilute hydrochloric
acid which is placed on a reading balance. The mass of the beaker and its contents is recorded every
two minutes, as shown in the table.
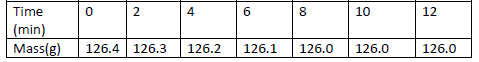
(a) Why is there a continuous loss of mass of the reaction mixture.
(b) Write an equation for the reaction taking place.
(c ) State two different ways by which the reaction could have been made more rapid.
(d) Why does the mass remain constant after 8 minutes.
(e) State the observations that would be made if a few drops of silver nitrate solution
Was added to 1cm3 of the resulting solution followed by ammonia solution.
Date posted: May 17, 2019. Answers (1)
- The standard electrode potentials for some half cells are given below.(Solved)
The standard electrode potentials for some half cells are given below.
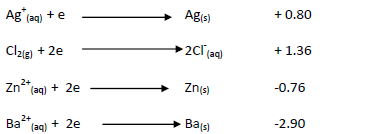
i)Arrange the metals in order of reactivity.
ii)Calculate the E.M.F of the cell shown below.

iii) What would happen if a cell with chlorine and zinc ions, the anode was made
of zinc. Explain your answer.
Date posted: May 17, 2019. Answers (1)
- The set-up below represents electrolysis of dilute sulphuric (VI) acid.(Solved)
The set-up below represents electrolysis of dilute sulphuric (VI) acid.
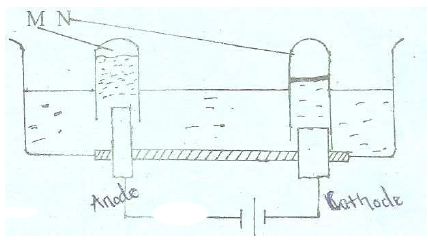
(a) Identify gases M and N
(b) Write an ionic equation for the production of gas M.
(c ) At what electrode does reduction take place. Explain your answer.
(d) State the most suitable electrodes that can be used in this experiment.
Explain your answer.
Date posted: May 17, 2019. Answers (1)
- Give the IUPAC names of the following compounds.(Solved)
Give the IUPAC names of the following compounds.

Date posted: May 17, 2019. Answers (1)