-
The set up below was used to prepare chlorine gas. (i) Identify solid M (ii) What is the role of water in the experiment?.....
(Solved)
The set up below was used to prepare chlorine gas.

(i) Identify solid M
(ii) What is the role of water in the experiment?
(iii) Complete the set up to show how dry chlorine gas can be collected
(iv) Write a chemical equation to show how chlorine gas is formed.
Date posted:
May 17, 2019
.
Answers (1)
-
The flow chart below summarizes the process of extraction of lead from a chief ore.
(Solved)
The flow chart below summarizes the process of extraction of lead from a chief ore.

(i) Identify process T
(ii) Give the name of:
Gas N
Solid F
(iii) Give two functions of CaCO3 in the extraction process.
(iv) Write an equation to show how waste product J is formed.
Date posted:
May 17, 2019
.
Answers (1)
-
A sealed glass tube containing 250 cm3 of nitrogen gas at r.t.p was immersed in boiling water. Calculate the pressure inside the tube if the...
(Solved)
A sealed glass tube containing 250 cm3 of nitrogen gas at r.t.p was immersed in boiling water. Calculate the pressure inside the tube if the volume of the gas does not change due to expansion of glass. (Room pressure=760mmHg, room temperature=298K).
Date posted:
May 17, 2019
.
Answers (1)
-
A solution of bromine in methyl benzene turns colourless when butane gas is passed through it.
(Solved)
A solution of bromine in methyl benzene turns colourless when butane gas is passed through it.
(a) What type of reaction takes place?
(b) Write equation of the reaction which takes place.
Date posted:
May 17, 2019
.
Answers (1)
-
60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide...
(Solved)
60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide (NO2) to diffuse under the same conditions. (O=16,N=14).
Date posted:
May 17, 2019
.
Answers (1)
-
The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that...
(Solved)
The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that follow.
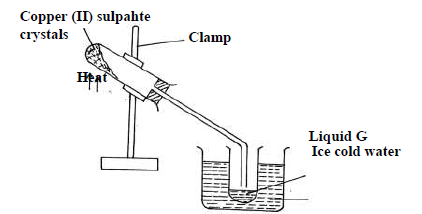
(a) Why is boiling tube slanted as shown?
(b) What is observed in the boiling tube.
(c) Identify liquid G.
Date posted:
May 17, 2019
.
Answers (1)
-
Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.
(Solved)
Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.
(i) Zinc + dilute nitric acid.
(ii) Lead + dilute hydrochloric acid.
(iii) Potassium + dilute sulphuric acid.
Date posted:
May 17, 2019
.
Answers (1)
-
Describe a simple test that can be carried out in the laboratory to distinguish between manganese (IV) oxide and copper (II) oxide.
(Solved)
Describe a simple test that can be carried out in the laboratory to distinguish between manganese (IV) oxide and copper (II) oxide.
Date posted:
May 17, 2019
.
Answers (1)
-
In an experiment to determine the molar heat of neutralization of hydrochloric acid
with sodium hydroride, students of Furaha Secondary school reacted 100cm3 of 1M
hydrochloric acid...
(Solved)
In an experiment to determine the molar heat of neutralization of hydrochloric acid
with sodium hydroride, students of Furaha Secondary school reacted 100cm3 of 1M
hydrochloric acid with 50cm3 of 2M sodium hydroxide solution. They obtained the following results.
Initial temperature of acid = 25.00C
Initial temperature of base = 25.00C
Highest temperature reached
With the acid – alkali mixture = 34.00C
(a) Write an ionic equation for the neutralization reaction between hydrochloric acid
and sodium hydroxide.
(b ) Calculate :
(i) The change in temperature.
(ii) The amount of heat produced during the reaction.
( Specific heat capacity of solution = 4.2 kJkg-1k-1 )
(iii) The molar heat of neutralization of sodium hydroxide.
(c) Write the thermochemical equation for the reaction.
(d) Draw an energy level diagram for the reaction.
Date posted:
May 17, 2019
.
Answers (1)
-
State two uses of carbon (IV) oxide.
(Solved)
State two uses of carbon (IV) oxide.
Date posted:
May 17, 2019
.
Answers (1)
-
A piece of marble chip ( calcium carbonate) is put in a beaker containing excess of dilute hydrochloric
acid which is placed on a reading balance....
(Solved)
A piece of marble chip ( calcium carbonate) is put in a beaker containing excess of dilute hydrochloric
acid which is placed on a reading balance. The mass of the beaker and its contents is recorded every
two minutes, as shown in the table.
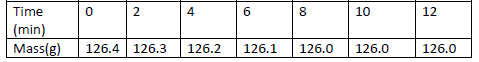
(a) Why is there a continuous loss of mass of the reaction mixture.
(b) Write an equation for the reaction taking place.
(c ) State two different ways by which the reaction could have been made more rapid.
(d) Why does the mass remain constant after 8 minutes.
(e) State the observations that would be made if a few drops of silver nitrate solution
Was added to 1cm3 of the resulting solution followed by ammonia solution.
Date posted:
May 17, 2019
.
Answers (1)
-
The set-up below represents electrolysis of dilute sulphuric (VI) acid.
(Solved)
The set-up below represents electrolysis of dilute sulphuric (VI) acid.
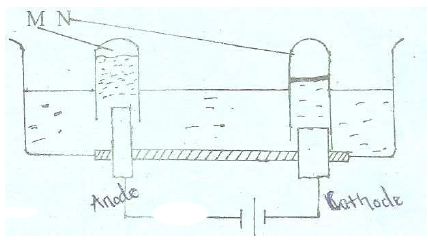
(a) Identify gases M and N
(b) Write an ionic equation for the production of gas M.
(c ) At what electrode does reduction take place. Explain your answer.
(d) State the most suitable electrodes that can be used in this experiment.
Explain your answer.
Date posted:
May 17, 2019
.
Answers (1)
-
The scheme below shows various reactions starting with ammonia. Study it and
answer the questions that follow.
(Solved)
The scheme below shows various reactions starting with ammonia. Study it and
answer the questions that follow.
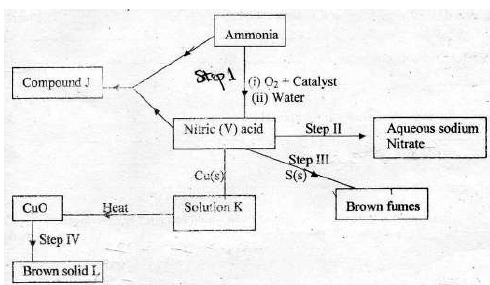
(i) List the raw materials used in the manufacture of ammonia.
(ii) What catalyst is used in step I ?
(iii) Write an equation for the reaction that occurs between ammonia and oxygen in
presence of the catalyst.
(iv) Identify the process in step II
(v) Using an appropriate equation, explain how the reaction in step III occurs ?
(vi) What should be added to solution K to form solid L ?
(vii) (a) (i) Write the formula of compound J.
(ii) Calculate the mass of compound J that would contain 14g of nitrogen
( H = 1, N = 14, O = 16 )
(b) State two advantages of ammonium phosphate over ammonium nitrate.
Date posted:
May 17, 2019
.
Answers (1)
-
The diagram below outlines industrial preparation of bleaching powder.
(Solved)
The diagram below outlines industrial preparation of bleaching powder.
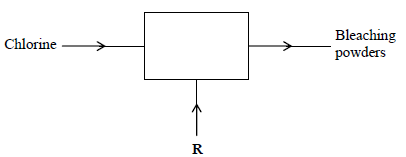
(i) Give the chemical name of bleaching powder
(ii) Identify substance R
(iii) Explain why water in which bleaching powder has been added needs a lot of soap
during washing.
Date posted:
May 17, 2019
.
Answers (1)
-
The reaction of water and calcium gave gas Q collected as in the diagram below
(Solved)
The reaction of water and calcium gave gas Q collected as in the diagram below
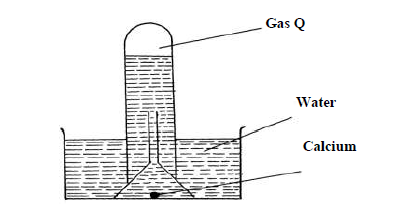
(i) Identify gas Q
(ii) Explain why the solution left after the reaction is a weak base.
Date posted:
May 17, 2019
.
Answers (1)
-
Dry hydrogen chloride gas was passed through heated iron wire as shown in the diagram
below
(Solved)
Dry hydrogen chloride gas was passed through heated iron wire as shown in the diagram
below
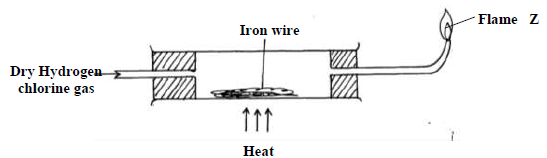
a) (i) How can the identity of the substance burning with flame Z be confirmed.
(ii) What is observed in combustion tube during the experiment?
(iii) Write the equation for the reaction taking place in the combustion tube.
(iv) Chlorine gas was passed over the product obtained in the combustion tube to give
another product Q
a) State one precaution that should be taken. Explain
b) Identify product Q
c) The total mass of product Q formed was found to be 5.3g. Calculate the volume of
chlorine gas used.
(Cl = 35.5, Fe= 56, Molar gas volume at room temperature = 2400cm3)
Date posted:
May 17, 2019
.
Answers (1)
-
The flow chart below outlines some of the process involved during extraction of copper.
(Solved)
The flow chart below outlines some of the process involved during extraction of copper.

(i) Write the formula of copper pyrite.
(ii) Name liquid T
(iii) Write equations for the reactions taking place in the 2nd roasting furnace.
(iv) Identify substance B and write equation for the reaction that take place in the smelting furnace.
(v) State the purpose of substance F.
Date posted:
May 17, 2019
.
Answers (1)
-
Study the scheme below and answer the questions that follow.
(Solved)
Study the scheme below and answer the questions that follow.

(i) Identify the product B.
(ii) Name the compounds C and E.
(iii) State the conditions for step 1
(iv) Write the equation for the reaction leading tot the formation of methane.
(v) State two industrial uses of methane.
(vi) Identify the reagent D
Date posted:
May 17, 2019
.
Answers (1)
-
The graph below was obtained from an experiment used to investigate the reaction
between Zinc granules and 2M hydrochloric acid.
(Solved)
The graph below was obtained from an experiment used to investigate the reaction
between Zinc granules and 2M hydrochloric acid.
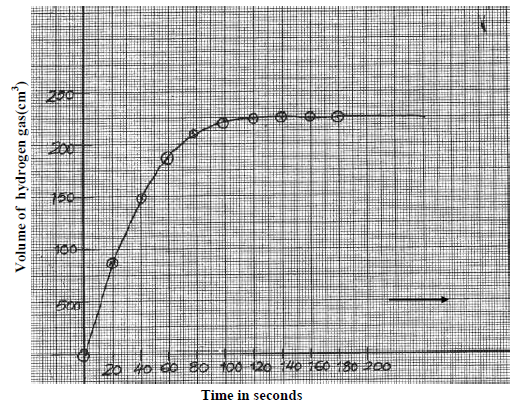
a) Calculate the rate of reaction when t = 60seconds.
b) Suggest how the rate of the above reaction can be reduced so that it can be studied more closely at the same temperature.
Date posted:
May 17, 2019
.
Answers (1)
-
Describe two observable characteristics of a luminous flame
(Solved)
Describe two observable characteristics of a luminous flame.
Date posted:
May 17, 2019
.
Answers (1)