- The extraction of aluminium from its ore takes place in two stages, purification stage
and electrolysis stage. The diagram below shows the set up for the...(Solved)
The extraction of aluminium from its ore takes place in two stages, purification stage
and electrolysis stage. The diagram below shows the set up for the electrolysis stage.
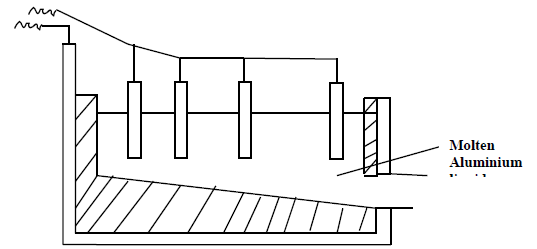
(a) Name the ore from which aluminium is extracted.
(b) Name one impurity which is removed at purification stage.
(c) Label on the diagram each of the following:
Anode
Cathode
Region containing the electrolyte
(d) The melting point of aluminium oxide is 20540C but electrolysis is done between 8000C -
9000C.
(i) Why is the electrolysis not carried out at 20540C.?
(ii) What is done to lower the temperature of the electrolysis cell to 8000C - 9000C?
(iii) The aluminium which is produced is tapped off as liquid. What does this imply
about its melting point?
(e) A typical electrolysis cell uses a current of 40000 ampheres. Calculate the mass (in kilograms) of aluminium produced in one hour.
Date posted: May 20, 2019. Answers (1)
- Study the diagram below and answer the questions that follow. When some hydrogen gas is allowed into the water....(Solved)
Study the diagram below and answer the questions that follow.
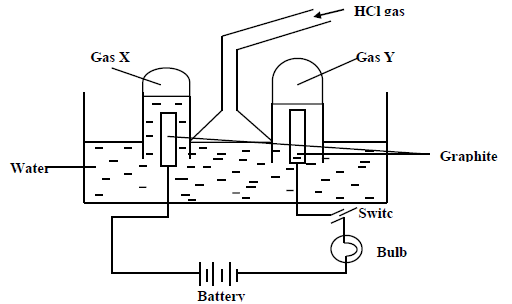
When some hydrogen gas is allowed into the water and the mixture stirred the bulb lights up
and gases X and Y are formed.
a) Name gas X
gas Y
(b) Write the chemical equations of how each of the gases is formed.
Gas X
Gas Y
(c) State any two uses of gas X.
(d) Explain why the bulb does not light before the hydrogen chloride gas is let into water.
Date posted: May 20, 2019. Answers (1)
- The table below gives information on four elements by letters A, B, C and D. Study it
and answer the questions that follow. The letters do...(Solved)
The table below gives information on four elements by letters A, B, C and D. Study it
and answer the questions that follow. The letters do not represent the actual
symbols of the elements.
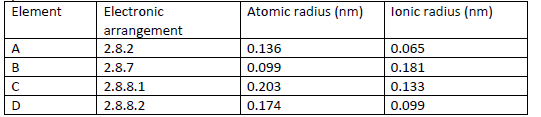
(a) Which two elements have two similar chemical properties? Explain.
(b) What is the most likely formula of the oxide of B?
(c) Which element is a non-metal?
(d) Which one of the elements is the strongest.
(i) Reducing agent?
(ii) Oxidising agent?
(e) Explain why ionic radius of D is less than that of C.
(f) Explain why the ionic radius of B is bigger than its atomic radius.
(g) Give the chemical family to which the element.
(i) A and D belong
(ii) B belong
(iii) C belong
(h) State any two uses of element B.
Date posted: May 20, 2019. Answers (1)
- The diagram below represents an incomplete paper chromatogram of pure dyes X, Y, Z and
mixture W.(Solved)
The diagram below represents an incomplete paper chromatogram of pure dyes X, Y, Z and
mixture W.
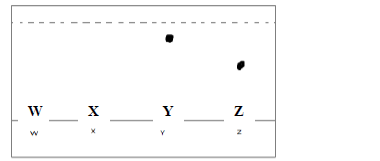
Mixture W contains dyes Y and Z only. Complete the chromatogram to show how mixture
W separates.
Date posted: May 20, 2019. Answers (1)
- The flow chart below outlines some of the process involved during extraction of copper.(Solved)
The flow chart below outlines some of the process involved during extraction of copper.

a) (i) Write the formula of copper pyrite.
(ii) Name liquid T
(iii) Write equations for the reactions taking place in the 2nd roasting furnace.
(iv) Identify substance B and write equation for the reaction that take place in the smelting furnace.
(v) State the purpose of substance F.
Date posted: May 20, 2019. Answers (1)
- The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the
following:(Solved)
The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the
following:

Date posted: May 17, 2019. Answers (1)
- Study the reduction potentials below.
(i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii)...(Solved)
Study the reduction potentials below.

(i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii) Write the cell diagram for the cell formed above.
Date posted: May 17, 2019. Answers (1)
- Study the diagram below and answer the questions which follow.(Solved)
Study the diagram below and answer the questions which follow.
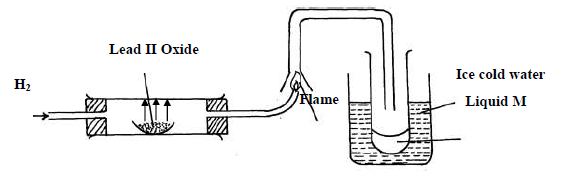
(i) State two observations made when hydrogen gas pass over hot lead II oxide.
(ii) Write the equation for the reaction which occurs in the combustion tube.
(iii) What property of hydrogen is shown in the experiment above
(iv) Identify liquid M.
(v) What type of reaction occurs when hydrogen gas reacts with butene?
(vi) State the condition required for the reaction (v) above
(vii) Apart from hydrogen peroxide, state two other reagents that can be used to prepare
oxygen gas.
(viii) Write an equation to show how hydrogen gas is formed from the reagents chosen in
(vii) above.
Date posted: May 17, 2019. Answers (1)
- What is atomicity?(Solved)
What is atomicity?
Date posted: May 17, 2019. Answers (1)
- Study the periodic grid below and answer the questions which follow. The letters do not
represent actual symbols of the elements.(Solved)
Study the periodic grid below and answer the questions which follow. The letters do not
represent actual symbols of the elements.
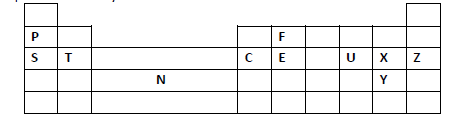
(i) To which category of elements does element N belong?
(ii) Compare the atomic radius of element U and X. Explain.
(iii) An ion A3- has a configuration of 2.8. Place element A on the grid above.
(iv) Which of the group 1 elements will require the greatest amount of energy to
remove the outermost electron. Explain.
(v) Why is element Z used in light bulbs?
(vi) Write the formula of the phosphate of element T.
(vii) State the type of structure found in the oxide of element F.
Date posted: May 17, 2019. Answers (1)
- A given amount of propane was used to heat one litre of water. The temperature of
the water rose from 25oC to 50.5oC. (S.H.C of water...(Solved)
A given amount of propane was used to heat one litre of water. The temperature of
the water rose from 25oC to 50.5oC. (S.H.C of water = 4.2J/g/k)
(i) Calculate the heat change for the reaction.
(ii) Find the mass of propane burnt (C=12, H=1)
Date posted: May 17, 2019. Answers (1)
- Study the heats of combustion shown below.(Solved)
Study the heats of combustion shown below.

Draw an energy cycle diagram linking heat of formation of propane with its heat of
combustion and the heat of combustion of the constituent elements.
Date posted: May 17, 2019. Answers (1)
- Define standard heat of combustion of a substance.(Solved)
Define standard heat of combustion of a substance.
Date posted: May 17, 2019. Answers (1)
- Study the diagram below. State and explain the observation made after sometime(Solved)
Study the diagram below.

State and explain the observation made after sometime.
Date posted: May 17, 2019. Answers (1)
- Give an equation to show how chlorine forms bleaching powder.(Solved)
Give an equation to show how chlorine forms bleaching powder.
Date posted: May 17, 2019. Answers (1)
- Chlorine reacts with cold dilute sodium hydroxide to form a bleaching agent.
Name the bleaching agent.(Solved)
Chlorine reacts with cold dilute sodium hydroxide to form a bleaching agent.
Name the bleaching agent.
Date posted: May 17, 2019. Answers (1)
- The set up below was used to prepare chlorine gas. (i) Identify solid M (ii) What is the role of water in the experiment?.....(Solved)
The set up below was used to prepare chlorine gas.

(i) Identify solid M
(ii) What is the role of water in the experiment?
(iii) Complete the set up to show how dry chlorine gas can be collected
(iv) Write a chemical equation to show how chlorine gas is formed.
Date posted: May 17, 2019. Answers (1)
- State one use of lead.(Solved)
State one use of lead.
Date posted: May 17, 2019. Answers (1)
- Pure lead can be obtained by electrolysis. Identify the anode and cathode for the
process.(Solved)
Pure lead can be obtained by electrolysis. Identify the anode and cathode for the
process.
Date posted: May 17, 2019. Answers (1)
- The flow chart below summarizes the process of extraction of lead from a chief ore.(Solved)
The flow chart below summarizes the process of extraction of lead from a chief ore.

(i) Identify process T
(ii) Give the name of:
Gas N
Solid F
(iii) Give two functions of CaCO3 in the extraction process.
(iv) Write an equation to show how waste product J is formed.
Date posted: May 17, 2019. Answers (1)