- 0.1mole of sodium chloride was dissolved in 100cm3 of water. Calculate the
concentration of this aqueous solution in grams per dm3 (Na=23, Cl=35.5).(Solved)
0.1mole of sodium chloride was dissolved in 100cm3 of water. Calculate the
concentration of this aqueous solution in grams per dm3 (Na=23, Cl=35.5).
Date posted: May 20, 2019. Answers (1)
- Study the scheme given below and answer the questions that follow (a) Name the reagents used in step I,II,III,IV,V(Solved)
Study the scheme given below and answer the questions that follow;
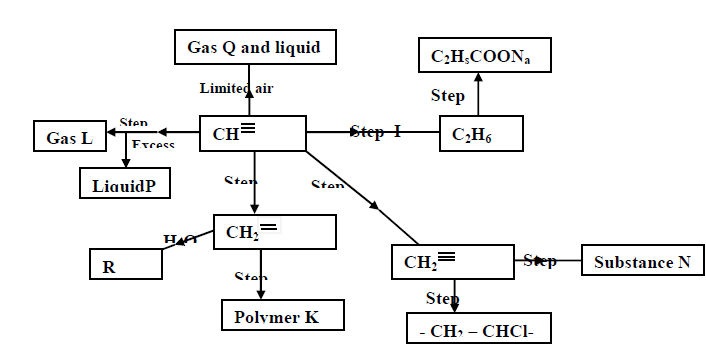
a) Name the reagents used in step I,II,III,IV,V:
b) Identify substances L,P,Q,R,K and N:
Date posted: May 20, 2019. Answers (1)
- State and explain the effect of the following on the equilibrium of the reaction indicated
below.(Solved)
State and explain the effect of the following on the equilibrium of the reaction indicated
below.
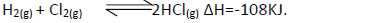
(i) Increase in pressure.
(ii) Increase in temperature.
(iii) Removal of chlorine gas.
Date posted: May 20, 2019. Answers (1)
- Bromine reacts with hydrogen to form hydrogen bromide gas as shown below:
(i) Determine the molar heat of the above reaction.
(ii) Write the equation for...(Solved)
Bromine reacts with hydrogen to form hydrogen bromide gas as shown below:
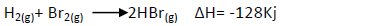
(i) Determine the molar heat of the above reaction.
(ii) Write the equation for the above case that show the molar heat of formation of
hydrogen bromide gas.
Date posted: May 20, 2019. Answers (1)
- A mass of 56g a saturated solution of salt X at 250C yield 14g of the solid when
evaporated to dryness. What is the solubility of...(Solved)
A mass of 56g a saturated solution of salt X at 250C yield 14g of the solid when
evaporated to dryness. What is the solubility of the salt at 250C.
Date posted: May 20, 2019. Answers (1)
- Below is a list of potential differences obtained when metals X, Y, Z, K and L are used in the
following electrochemical cell.
Metal(s)/metal ion (aq)//copper(ii)ions/copper.(Solved)
Below is a list of potential differences obtained when metals X, Y, Z, K and L are used in the
following electrochemical cell.
Metal(s)/metal ion (aq)//copper(ii)ions/copper.
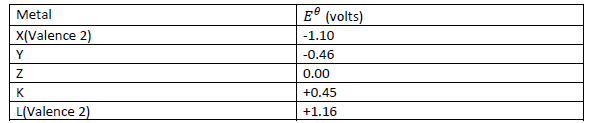
a) What is metal Z? Explain.
(b) Which two of the above metals in an electrochemical cell would produce the largest
electromotive force across the cell? What is this electromotive force?
(c) Write the cell equation of the pair of metals that will produce the largest potential
difference.
(d) Write the cell equation of the pair of metals that will produce the largest negative potential
difference. Determine this voltage.
Date posted: May 20, 2019. Answers (1)
- A natural element represented by letter Y has two types of atoms. The composition
of the particles is as summarized below:(Solved)
A natural element represented by letter Y has two types of atoms. The composition
of the particles is as summarized below:

(a) Complete the missing number.
(b) What is the name assigned to these two types of atoms?
(c) Which atom has the least percentage of abundance?
(d) Calculate the relative atomic mass of Y.
(e) Explain what is made by nuclear particles giving examples where possible.
Date posted: May 20, 2019. Answers (1)
- The extraction of aluminium from its ore takes place in two stages, purification stage
and electrolysis stage. The diagram below shows the set up for the...(Solved)
The extraction of aluminium from its ore takes place in two stages, purification stage
and electrolysis stage. The diagram below shows the set up for the electrolysis stage.
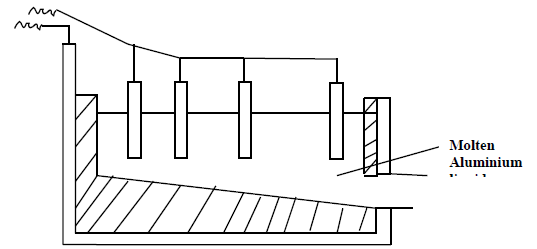
(a) Name the ore from which aluminium is extracted.
(b) Name one impurity which is removed at purification stage.
(c) Label on the diagram each of the following:
Anode
Cathode
Region containing the electrolyte
(d) The melting point of aluminium oxide is 20540C but electrolysis is done between 8000C -
9000C.
(i) Why is the electrolysis not carried out at 20540C.?
(ii) What is done to lower the temperature of the electrolysis cell to 8000C - 9000C?
(iii) The aluminium which is produced is tapped off as liquid. What does this imply
about its melting point?
(e) A typical electrolysis cell uses a current of 40000 ampheres. Calculate the mass (in kilograms) of aluminium produced in one hour.
Date posted: May 20, 2019. Answers (1)
- Study the diagram below and answer the questions that follow. When some hydrogen gas is allowed into the water....(Solved)
Study the diagram below and answer the questions that follow.
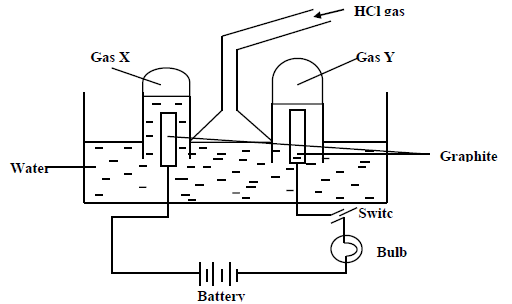
When some hydrogen gas is allowed into the water and the mixture stirred the bulb lights up
and gases X and Y are formed.
a) Name gas X
gas Y
(b) Write the chemical equations of how each of the gases is formed.
Gas X
Gas Y
(c) State any two uses of gas X.
(d) Explain why the bulb does not light before the hydrogen chloride gas is let into water.
Date posted: May 20, 2019. Answers (1)
- The table below gives information on four elements by letters A, B, C and D. Study it
and answer the questions that follow. The letters do...(Solved)
The table below gives information on four elements by letters A, B, C and D. Study it
and answer the questions that follow. The letters do not represent the actual
symbols of the elements.
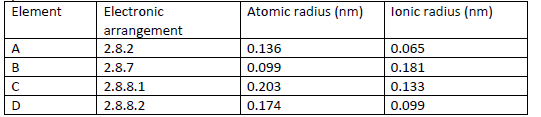
(a) Which two elements have two similar chemical properties? Explain.
(b) What is the most likely formula of the oxide of B?
(c) Which element is a non-metal?
(d) Which one of the elements is the strongest.
(i) Reducing agent?
(ii) Oxidising agent?
(e) Explain why ionic radius of D is less than that of C.
(f) Explain why the ionic radius of B is bigger than its atomic radius.
(g) Give the chemical family to which the element.
(i) A and D belong
(ii) B belong
(iii) C belong
(h) State any two uses of element B.
Date posted: May 20, 2019. Answers (1)
- The diagram below represents an incomplete paper chromatogram of pure dyes X, Y, Z and
mixture W.(Solved)
The diagram below represents an incomplete paper chromatogram of pure dyes X, Y, Z and
mixture W.
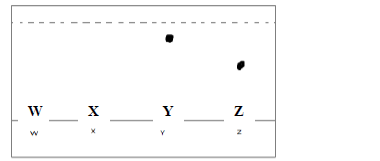
Mixture W contains dyes Y and Z only. Complete the chromatogram to show how mixture
W separates.
Date posted: May 20, 2019. Answers (1)
- The flow chart below outlines some of the process involved during extraction of copper.(Solved)
The flow chart below outlines some of the process involved during extraction of copper.

a) (i) Write the formula of copper pyrite.
(ii) Name liquid T
(iii) Write equations for the reactions taking place in the 2nd roasting furnace.
(iv) Identify substance B and write equation for the reaction that take place in the smelting furnace.
(v) State the purpose of substance F.
Date posted: May 20, 2019. Answers (1)
- The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the
following:(Solved)
The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the
following:

Date posted: May 17, 2019. Answers (1)
- Study the reduction potentials below.
(i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii)...(Solved)
Study the reduction potentials below.

(i) Identify the weakest oxidizing agent.
(ii) Calculate the e.m.f of the cell that would produce the highest output of voltage.
(iii) Write the cell diagram for the cell formed above.
Date posted: May 17, 2019. Answers (1)
- Study the diagram below and answer the questions which follow.(Solved)
Study the diagram below and answer the questions which follow.
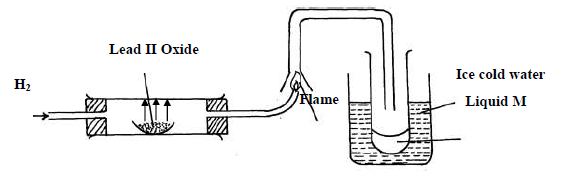
(i) State two observations made when hydrogen gas pass over hot lead II oxide.
(ii) Write the equation for the reaction which occurs in the combustion tube.
(iii) What property of hydrogen is shown in the experiment above
(iv) Identify liquid M.
(v) What type of reaction occurs when hydrogen gas reacts with butene?
(vi) State the condition required for the reaction (v) above
(vii) Apart from hydrogen peroxide, state two other reagents that can be used to prepare
oxygen gas.
(viii) Write an equation to show how hydrogen gas is formed from the reagents chosen in
(vii) above.
Date posted: May 17, 2019. Answers (1)
- What is atomicity?(Solved)
What is atomicity?
Date posted: May 17, 2019. Answers (1)
- Study the periodic grid below and answer the questions which follow. The letters do not
represent actual symbols of the elements.(Solved)
Study the periodic grid below and answer the questions which follow. The letters do not
represent actual symbols of the elements.
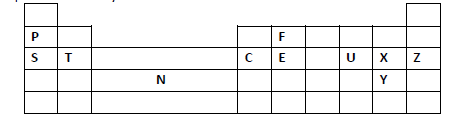
(i) To which category of elements does element N belong?
(ii) Compare the atomic radius of element U and X. Explain.
(iii) An ion A3- has a configuration of 2.8. Place element A on the grid above.
(iv) Which of the group 1 elements will require the greatest amount of energy to
remove the outermost electron. Explain.
(v) Why is element Z used in light bulbs?
(vi) Write the formula of the phosphate of element T.
(vii) State the type of structure found in the oxide of element F.
Date posted: May 17, 2019. Answers (1)
- A given amount of propane was used to heat one litre of water. The temperature of
the water rose from 25oC to 50.5oC. (S.H.C of water...(Solved)
A given amount of propane was used to heat one litre of water. The temperature of
the water rose from 25oC to 50.5oC. (S.H.C of water = 4.2J/g/k)
(i) Calculate the heat change for the reaction.
(ii) Find the mass of propane burnt (C=12, H=1)
Date posted: May 17, 2019. Answers (1)
- Study the heats of combustion shown below.(Solved)
Study the heats of combustion shown below.

Draw an energy cycle diagram linking heat of formation of propane with its heat of
combustion and the heat of combustion of the constituent elements.
Date posted: May 17, 2019. Answers (1)
- Define standard heat of combustion of a substance.(Solved)
Define standard heat of combustion of a substance.
Date posted: May 17, 2019. Answers (1)