- Study the flow chart below and answer the questions that follows (i) Give the reagents and conditions for step II to occur(Solved)
Study the flow chart below and answer the questions that follows.
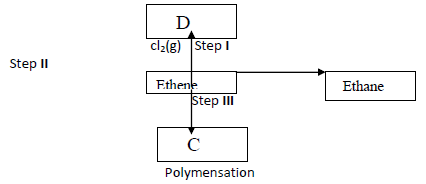
(i) Give the reagents and conditions for step II to occur
(ii) Give the industrial importance of step II.
(iii) Name the compound C
Date posted: May 20, 2019. Answers (1)
- Draw the structural formulae of the following compounds
(i) 2 methyl propene
(ii) Butan –2-ol
(iii) 2-3-dimethyl Butane(Solved)
Draw the structural formulae of the following compounds
(i) 2 methyl propene
(ii) Butan –2-ol
(iii) 2-3-dimethyl Butane
Date posted: May 20, 2019. Answers (1)
- In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited...(Solved)
In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited on the spoon ( IF =96500 coulombs,Ag=108)
Date posted: May 20, 2019. Answers (1)
- Write one structural formulae of
(i) Methanol.
(ii) Methanoic acid.
(b) Write the equation for the reaction between methanoic acid and sodium hydroxide
(c) Name the product formed when...(Solved)
Write one structural formulae of
(i) Methanol.
(ii) Methanoic acid.
(b) Write the equation for the reaction between methanoic acid and sodium hydroxide
(c) Name the product formed when methanol reacts with methanoic acid.
(d) State one condition necessary for the reaction in (c) to take place
Date posted: May 20, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow (a) Identify W and P....(Solved)
Study the flow chart below and answer the questions that follow
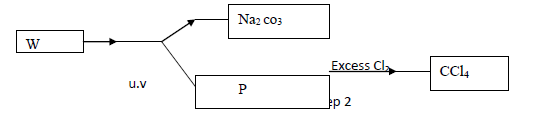
(a) Identify W and P.
(b) What name is given to the type of halogenation reaction in step 2.
Date posted: May 20, 2019. Answers (1)
- The table below gives reduction potentials obtained when the half-cells for each of the
elements represented by A, B, C, D and E were connected to...(Solved)
The table below gives reduction potentials obtained when the half-cells for each of the
elements represented by A, B, C, D and E were connected to a copper half-cell as the reference
electrode.
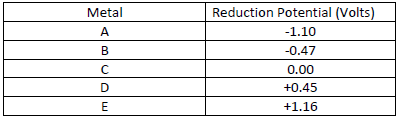
(a) What is element C likely to be? Give a reason.
(b) Which of the metals cannot be displaced from the solution of its salt by any other metal in
the table. Give a reason.
Date posted: May 20, 2019. Answers (1)
- The table below gives information on four elements represented by K L M & N. Study it and answer the questions that follow. The letters...(Solved)
The table below gives information on four elements represented by K L M & N. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
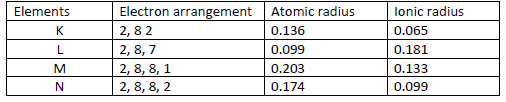
Which two elements have similar chemical properties? Explain
Date posted: May 20, 2019. Answers (1)
- Study the diagram below and answer the questions that follow. When some hydrogen chloride gas is allowed into water....(Solved)
Study the diagram below and answer the questions that follow.
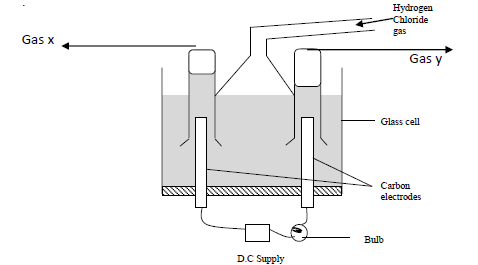
When some hydrogen chloride gas is allowed into water and the mixture stirred, the bulb lights and gasses X and Y are formed.
(a) Name
(i) Gas X
(ii) Gas Y
(b) Explain why the bulb does not light before the chloride gas is let into the water
Date posted: May 20, 2019. Answers (1)
- For each of the following experiments, give the observations, and the type of change that
occurs (Physical or chemical)(Solved)
For each of the following experiments, give the observations, and the type of change that
occurs (Physical or chemical)
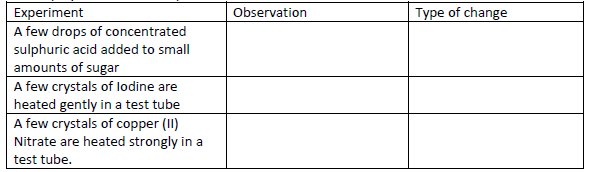
Date posted: May 20, 2019. Answers (1)
- The structure of ammonium ion is shown below. Name the type of bond represented in the diagram by N-----> H(Solved)
The structure of ammonium ion is shown below
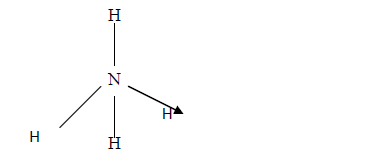
Name the type of bond represented in the diagram by N-----> H
Date posted: May 20, 2019. Answers (1)
- Give a reason why ammonia gas is highly soluble in water.(Solved)
Give a reason why ammonia gas is highly soluble in water.
Date posted: May 20, 2019. Answers (1)
- Define the following terms
(a) Isotopes
(b) Mass number
(c) Isobars(Solved)
Define the following terms
(a) Isotopes
(b) Mass number
(c) Isobars
Date posted: May 20, 2019. Answers (1)
- Draw reaction cycles for the cases shown below.(Solved)
Draw reaction cycles for the cases shown below.
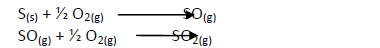
Date posted: May 20, 2019. Answers (1)
- 0.1mole of sodium chloride was dissolved in 100cm3 of water. Calculate the
concentration of this aqueous solution in grams per dm3 (Na=23, Cl=35.5).(Solved)
0.1mole of sodium chloride was dissolved in 100cm3 of water. Calculate the
concentration of this aqueous solution in grams per dm3 (Na=23, Cl=35.5).
Date posted: May 20, 2019. Answers (1)
- Study the scheme given below and answer the questions that follow (a) Name the reagents used in step I,II,III,IV,V(Solved)
Study the scheme given below and answer the questions that follow;
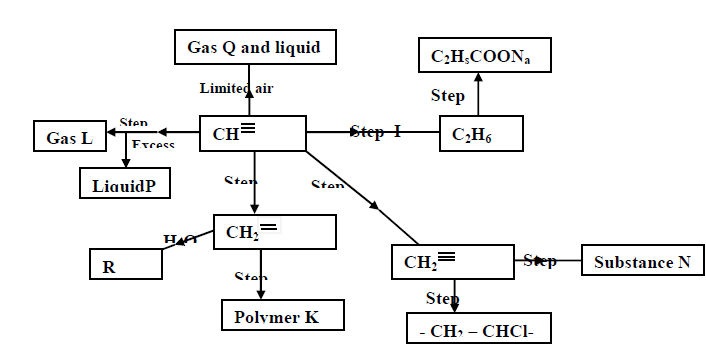
a) Name the reagents used in step I,II,III,IV,V:
b) Identify substances L,P,Q,R,K and N:
Date posted: May 20, 2019. Answers (1)
- State and explain the effect of the following on the equilibrium of the reaction indicated
below.(Solved)
State and explain the effect of the following on the equilibrium of the reaction indicated
below.
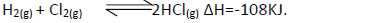
(i) Increase in pressure.
(ii) Increase in temperature.
(iii) Removal of chlorine gas.
Date posted: May 20, 2019. Answers (1)
- Bromine reacts with hydrogen to form hydrogen bromide gas as shown below:
(i) Determine the molar heat of the above reaction.
(ii) Write the equation for...(Solved)
Bromine reacts with hydrogen to form hydrogen bromide gas as shown below:
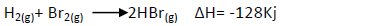
(i) Determine the molar heat of the above reaction.
(ii) Write the equation for the above case that show the molar heat of formation of
hydrogen bromide gas.
Date posted: May 20, 2019. Answers (1)
- A mass of 56g a saturated solution of salt X at 250C yield 14g of the solid when
evaporated to dryness. What is the solubility of...(Solved)
A mass of 56g a saturated solution of salt X at 250C yield 14g of the solid when
evaporated to dryness. What is the solubility of the salt at 250C.
Date posted: May 20, 2019. Answers (1)
- Below is a list of potential differences obtained when metals X, Y, Z, K and L are used in the
following electrochemical cell.
Metal(s)/metal ion (aq)//copper(ii)ions/copper.(Solved)
Below is a list of potential differences obtained when metals X, Y, Z, K and L are used in the
following electrochemical cell.
Metal(s)/metal ion (aq)//copper(ii)ions/copper.
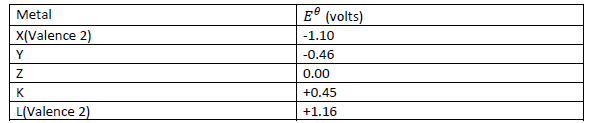
a) What is metal Z? Explain.
(b) Which two of the above metals in an electrochemical cell would produce the largest
electromotive force across the cell? What is this electromotive force?
(c) Write the cell equation of the pair of metals that will produce the largest potential
difference.
(d) Write the cell equation of the pair of metals that will produce the largest negative potential
difference. Determine this voltage.
Date posted: May 20, 2019. Answers (1)
- A natural element represented by letter Y has two types of atoms. The composition
of the particles is as summarized below:(Solved)
A natural element represented by letter Y has two types of atoms. The composition
of the particles is as summarized below:

(a) Complete the missing number.
(b) What is the name assigned to these two types of atoms?
(c) Which atom has the least percentage of abundance?
(d) Calculate the relative atomic mass of Y.
(e) Explain what is made by nuclear particles giving examples where possible.
Date posted: May 20, 2019. Answers (1)