- The table below shows tests carried out in a separate sample of water drawn from a well and
results obtained.(Solved)
The table below shows tests carried out in a separate sample of water drawn from a well and
results obtained.

Identify the cation and anion present in the water
Cation
Anion
Date posted: May 20, 2019. Answers (1)
- Identify the species that acts as a base in the reverse reaction given below. Give a reason.(Solved)
Identify the species that acts as a base in the reverse reaction given below. Give a reason.
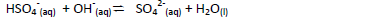
Date posted: May 20, 2019. Answers (1)
- Below is a flow chart. Study it and answer the questions that follow (i) Name the process in step I (ii) Name compound R Reagent Y....(Solved)
Below is a flow chart. Study it and answer the questions that follow: -
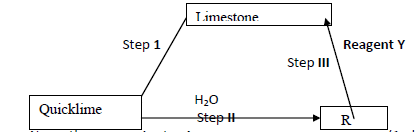
(i) Name the process in step I
(ii) Name compound R
Reagent Y
(iii) Write equation for the reaction in step II
(c) Explain why 0.1 M hydrochloric acid has a pH of 1 while 0.1M ethanoic acid has a pH of 3
Date posted: May 20, 2019. Answers (1)
- Define the term duplet.(Solved)
Define the term duplet.
Date posted: May 20, 2019. Answers (1)
- State one environmental hazard that is caused during extraction of sodium metal.(Solved)
State one environmental hazard that is caused during extraction of sodium metal.
Date posted: May 20, 2019. Answers (1)
- Calculate the volume of hydrogen gas produced at s.t.p when 1.15g of sodium metal
react with water. (Na=23, molar gas volume=22400cm3)(Solved)
Calculate the volume of hydrogen gas produced at s.t.p when 1.15g of sodium metal
react with water. (Na=23, molar gas volume=22400cm3)
Date posted: May 20, 2019. Answers (1)
- What is the function of calcium chloride during extraction of sodium metal?(Solved)
What is the function of calcium chloride during extraction of sodium metal?
Date posted: May 20, 2019. Answers (1)
- Name the process in which sodium metal is extracted.(Solved)
Name the process in which sodium metal is extracted.
Date posted: May 20, 2019. Answers (1)
- The setup below was used to prepare and collect a dry sample of gas X. Study it and answer
the questions that follow.(Solved)
The setup below was used to prepare and collect a dry sample of gas X. Study it and answer
the questions that follow.
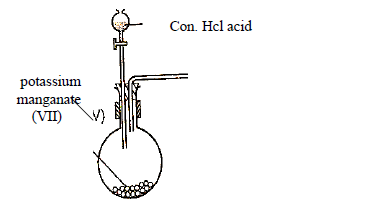
(a) Identify gas X
(b) Complete the setup to show how gas X is dried and collected.
(c) Write an equation for the above reaction.
(d) An aqueous solution of zinc sulphate is electrolysed using platinum electrodes. State
and explain what happens to the concentration of zinc sulphate
(e) State the ratio of the products of the anode and cathode using the equations
(f) Give one use of electrolysis
(g) What is anodization of aluminium
Date posted: May 20, 2019. Answers (1)
- Study the flow chart below and answer the questions that follows (i) Give the reagents and conditions for step II to occur(Solved)
Study the flow chart below and answer the questions that follows.
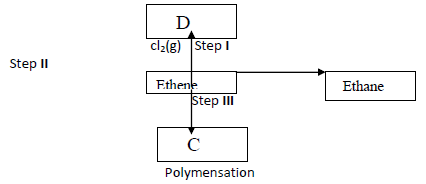
(i) Give the reagents and conditions for step II to occur
(ii) Give the industrial importance of step II.
(iii) Name the compound C
Date posted: May 20, 2019. Answers (1)
- Draw the structural formulae of the following compounds
(i) 2 methyl propene
(ii) Butan –2-ol
(iii) 2-3-dimethyl Butane(Solved)
Draw the structural formulae of the following compounds
(i) 2 methyl propene
(ii) Butan –2-ol
(iii) 2-3-dimethyl Butane
Date posted: May 20, 2019. Answers (1)
- In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited...(Solved)
In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited on the spoon ( IF =96500 coulombs,Ag=108)
Date posted: May 20, 2019. Answers (1)
- Write one structural formulae of
(i) Methanol.
(ii) Methanoic acid.
(b) Write the equation for the reaction between methanoic acid and sodium hydroxide
(c) Name the product formed when...(Solved)
Write one structural formulae of
(i) Methanol.
(ii) Methanoic acid.
(b) Write the equation for the reaction between methanoic acid and sodium hydroxide
(c) Name the product formed when methanol reacts with methanoic acid.
(d) State one condition necessary for the reaction in (c) to take place
Date posted: May 20, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow (a) Identify W and P....(Solved)
Study the flow chart below and answer the questions that follow
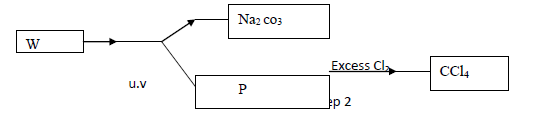
(a) Identify W and P.
(b) What name is given to the type of halogenation reaction in step 2.
Date posted: May 20, 2019. Answers (1)
- The table below gives reduction potentials obtained when the half-cells for each of the
elements represented by A, B, C, D and E were connected to...(Solved)
The table below gives reduction potentials obtained when the half-cells for each of the
elements represented by A, B, C, D and E were connected to a copper half-cell as the reference
electrode.
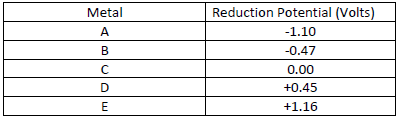
(a) What is element C likely to be? Give a reason.
(b) Which of the metals cannot be displaced from the solution of its salt by any other metal in
the table. Give a reason.
Date posted: May 20, 2019. Answers (1)
- The table below gives information on four elements represented by K L M & N. Study it and answer the questions that follow. The letters...(Solved)
The table below gives information on four elements represented by K L M & N. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
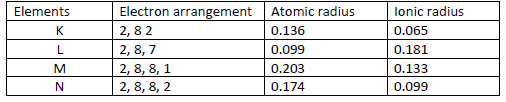
Which two elements have similar chemical properties? Explain
Date posted: May 20, 2019. Answers (1)
- Study the diagram below and answer the questions that follow. When some hydrogen chloride gas is allowed into water....(Solved)
Study the diagram below and answer the questions that follow.
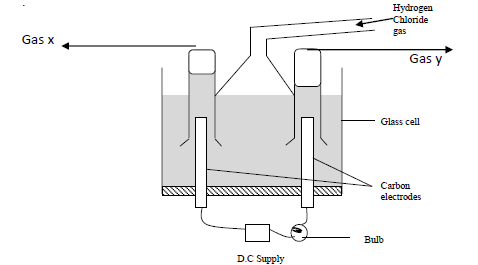
When some hydrogen chloride gas is allowed into water and the mixture stirred, the bulb lights and gasses X and Y are formed.
(a) Name
(i) Gas X
(ii) Gas Y
(b) Explain why the bulb does not light before the chloride gas is let into the water
Date posted: May 20, 2019. Answers (1)
- For each of the following experiments, give the observations, and the type of change that
occurs (Physical or chemical)(Solved)
For each of the following experiments, give the observations, and the type of change that
occurs (Physical or chemical)
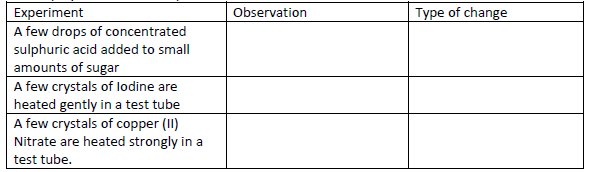
Date posted: May 20, 2019. Answers (1)
- The structure of ammonium ion is shown below. Name the type of bond represented in the diagram by N-----> H(Solved)
The structure of ammonium ion is shown below
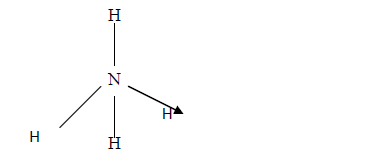
Name the type of bond represented in the diagram by N-----> H
Date posted: May 20, 2019. Answers (1)
- Give a reason why ammonia gas is highly soluble in water.(Solved)
Give a reason why ammonia gas is highly soluble in water.
Date posted: May 20, 2019. Answers (1)