Add NaOH(aq) to the solution of Fe2+ and Fe3+ separately until in excess.
Fe2+- dirt green precipitate. is formed insoluble in excess.
Fe3+ - yellow red-brown precipitate. formed insoluble in excess
sharon kalunda answered the question on May 20, 2019 at 10:56
- The diagram below shows two types of detergents which one of these detergents is a soap?
Give a reason for your choice.(Solved)
The diagram below shows two types of detergents which one of these detergents is a soap?
Give a reason for your choice.
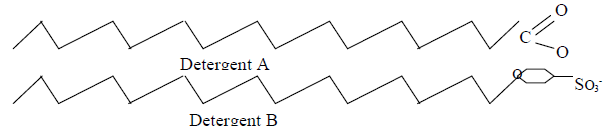
Date posted: May 20, 2019. Answers (1)
- Below is a table of first five alkanes and their boiling points.(Solved)
Below is a table of first five alkanes and their boiling points.

What is the state of pentane at room temperature ( 25oC)? Give a reasons.
Date posted: May 20, 2019. Answers (1)
- Hydrogen peroxide decomposes according to the equation shown below.(Solved)
Hydrogen peroxide decomposes according to the equation shown below.
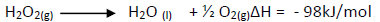
8.5g of hydrogen peroxide contained in 100cm3 of solution with water were completely
decomposed.Calculate the rise in temperature due to the reaction.(specific heat capacity on water = 4.25
Date posted: May 20, 2019. Answers (1)
- In a closed system an equilibrium exists between nitrogen(IV) oxide and dinitrogen tetraoxide
as shown in the equation.(Solved)
In a closed system an equilibrium exists between nitrogen(IV) oxide and dinitrogen tetraoxide
as shown in the equation.
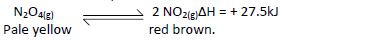
State and explain the observation made when a glass syringe containing the equilibrium mixture is immersed in ice-cold water.
Date posted: May 20, 2019. Answers (1)
- A concentrated solution of sulphuric (VI) acid contain 72.5% sulphuric (VI) acid. If the density
of the acid is 1.8g/cm3 determine the molarity of the acid...(Solved)
A concentrated solution of sulphuric (VI) acid contain 72.5% sulphuric (VI) acid. If the density
of the acid is 1.8g/cm3 determine the molarity of the acid solution. (H= 1, O=16, S = 32)
Date posted: May 20, 2019. Answers (1)
- What is the colour of the following?(Solved)
What is the colour of the following?


Date posted: May 20, 2019. Answers (1)
- Chlorine reacts with methane as shown below.
(a) What condition is necessary for this reaction to take place?
(b) Identify the bonds which are broken and those...(Solved)
Chlorine reacts with methane as shown below.
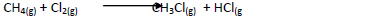
(a) What condition is necessary for this reaction to take place?
(b) Identify the bonds which are broken and those that are formed.
(i) Bonds broken.
(ii) Bonds formed.
Date posted: May 20, 2019. Answers (1)
- Hydrogen and Flourine react according to the equation. On the grid provided below, sketch the energy level diagram for the reverse reaction.(Solved)
Hydrogen and Flourine react according to the equation.
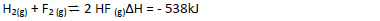
On the grid provided below, sketch the energy level diagram for the reverse reaction.
(b) Calculate the molar enthalpy of formation of HF.
Date posted: May 20, 2019. Answers (1)
- The table below shows tests carried out in a separate sample of water drawn from a well and
results obtained.(Solved)
The table below shows tests carried out in a separate sample of water drawn from a well and
results obtained.

Identify the cation and anion present in the water
Cation
Anion
Date posted: May 20, 2019. Answers (1)
- Identify the species that acts as a base in the reverse reaction given below. Give a reason.(Solved)
Identify the species that acts as a base in the reverse reaction given below. Give a reason.
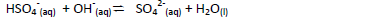
Date posted: May 20, 2019. Answers (1)
- Below is a flow chart. Study it and answer the questions that follow (i) Name the process in step I (ii) Name compound R Reagent Y....(Solved)
Below is a flow chart. Study it and answer the questions that follow: -
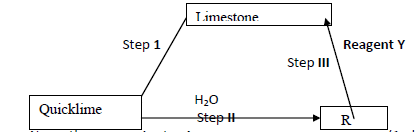
(i) Name the process in step I
(ii) Name compound R
Reagent Y
(iii) Write equation for the reaction in step II
(c) Explain why 0.1 M hydrochloric acid has a pH of 1 while 0.1M ethanoic acid has a pH of 3
Date posted: May 20, 2019. Answers (1)
- Define the term duplet.(Solved)
Define the term duplet.
Date posted: May 20, 2019. Answers (1)
- State one environmental hazard that is caused during extraction of sodium metal.(Solved)
State one environmental hazard that is caused during extraction of sodium metal.
Date posted: May 20, 2019. Answers (1)
- Calculate the volume of hydrogen gas produced at s.t.p when 1.15g of sodium metal
react with water. (Na=23, molar gas volume=22400cm3)(Solved)
Calculate the volume of hydrogen gas produced at s.t.p when 1.15g of sodium metal
react with water. (Na=23, molar gas volume=22400cm3)
Date posted: May 20, 2019. Answers (1)
- What is the function of calcium chloride during extraction of sodium metal?(Solved)
What is the function of calcium chloride during extraction of sodium metal?
Date posted: May 20, 2019. Answers (1)
- Name the process in which sodium metal is extracted.(Solved)
Name the process in which sodium metal is extracted.
Date posted: May 20, 2019. Answers (1)
- The setup below was used to prepare and collect a dry sample of gas X. Study it and answer
the questions that follow.(Solved)
The setup below was used to prepare and collect a dry sample of gas X. Study it and answer
the questions that follow.
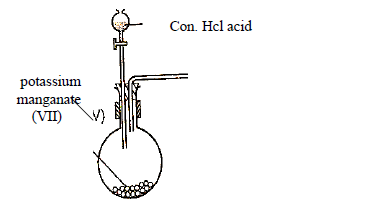
(a) Identify gas X
(b) Complete the setup to show how gas X is dried and collected.
(c) Write an equation for the above reaction.
(d) An aqueous solution of zinc sulphate is electrolysed using platinum electrodes. State
and explain what happens to the concentration of zinc sulphate
(e) State the ratio of the products of the anode and cathode using the equations
(f) Give one use of electrolysis
(g) What is anodization of aluminium
Date posted: May 20, 2019. Answers (1)
- Study the flow chart below and answer the questions that follows (i) Give the reagents and conditions for step II to occur(Solved)
Study the flow chart below and answer the questions that follows.
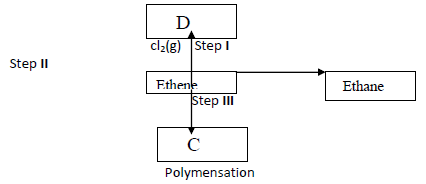
(i) Give the reagents and conditions for step II to occur
(ii) Give the industrial importance of step II.
(iii) Name the compound C
Date posted: May 20, 2019. Answers (1)
- Draw the structural formulae of the following compounds
(i) 2 methyl propene
(ii) Butan –2-ol
(iii) 2-3-dimethyl Butane(Solved)
Draw the structural formulae of the following compounds
(i) 2 methyl propene
(ii) Butan –2-ol
(iii) 2-3-dimethyl Butane
Date posted: May 20, 2019. Answers (1)
- In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited...(Solved)
In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited on the spoon ( IF =96500 coulombs,Ag=108)
Date posted: May 20, 2019. Answers (1)