– sodium floats because it is less dense
- Effervescence seen a gas is formed
- Hissing sound heard because hydrogen gas is formed
sharon kalunda answered the question on May 20, 2019 at 11:55
-
Raw rubber is heated with sulphur in the manufacture of natural rubber.
(Solved)
Raw rubber is heated with sulphur in the manufacture of natural rubber.
(i) What name is given to the process?
(ii Why is the process necessary.
Date posted:
May 20, 2019
.
Answers (1)
-
Below is a flow chart. Study it and answer the questions that follow (i) Name the process in step I (ii) Name compound R Reagent Y....
(Solved)
Below is a flow chart. Study it and answer the questions that follow: -
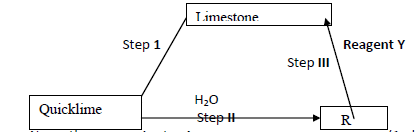
(i) Name the process in step I
(ii) Name compound R
Reagent Y
(iii) Write equation for the reaction in step II
(c) Explain why 0.1 M hydrochloric acid has a pH of 1 while 0.1M ethanoic acid has a pH of 3
Date posted:
May 20, 2019
.
Answers (1)
-
Calculate the volume of hydrogen gas produced at s.t.p when 1.15g of sodium metal
react with water. (Na=23, molar gas volume=22400cm3)
(Solved)
Calculate the volume of hydrogen gas produced at s.t.p when 1.15g of sodium metal
react with water. (Na=23, molar gas volume=22400cm3)
Date posted:
May 20, 2019
.
Answers (1)
-
What is the function of calcium chloride during extraction of sodium metal?
(Solved)
What is the function of calcium chloride during extraction of sodium metal?
Date posted:
May 20, 2019
.
Answers (1)
-
In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited...
(Solved)
In an experiment to electroplate a copper spoon with silver, a current of 0.5A was passed for 18 minutes. Calculate the amount of silver deposited on the spoon ( IF =96500 coulombs,Ag=108)
Date posted:
May 20, 2019
.
Answers (1)
-
Study the diagram below and answer the questions that follow. When some hydrogen chloride gas is allowed into water....
(Solved)
Study the diagram below and answer the questions that follow.
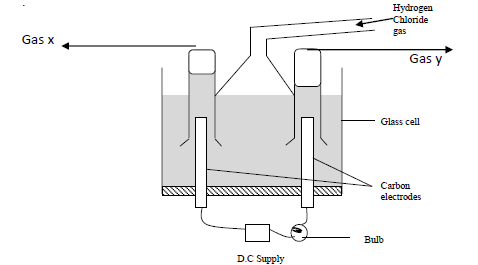
When some hydrogen chloride gas is allowed into water and the mixture stirred, the bulb lights and gasses X and Y are formed.
(a) Name
(i) Gas X
(ii) Gas Y
(b) Explain why the bulb does not light before the chloride gas is let into the water
Date posted:
May 20, 2019
.
Answers (1)
-
The structure of ammonium ion is shown below. Name the type of bond represented in the diagram by N-----> H
(Solved)
The structure of ammonium ion is shown below
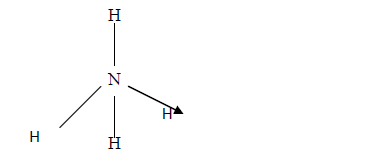
Name the type of bond represented in the diagram by N-----> H
Date posted:
May 20, 2019
.
Answers (1)
-
Give a reason why ammonia gas is highly soluble in water.
(Solved)
Give a reason why ammonia gas is highly soluble in water.
Date posted:
May 20, 2019
.
Answers (1)
-
A mass of 56g a saturated solution of salt X at 250C yield 14g of the solid when
evaporated to dryness. What is the solubility of...
(Solved)
A mass of 56g a saturated solution of salt X at 250C yield 14g of the solid when
evaporated to dryness. What is the solubility of the salt at 250C.
Date posted:
May 20, 2019
.
Answers (1)
-
The extraction of aluminium from its ore takes place in two stages, purification stage
and electrolysis stage. The diagram below shows the set up for the...
(Solved)
The extraction of aluminium from its ore takes place in two stages, purification stage
and electrolysis stage. The diagram below shows the set up for the electrolysis stage.
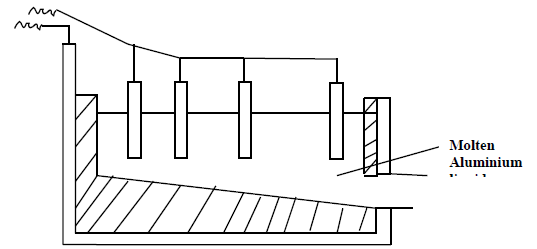
(a) Name the ore from which aluminium is extracted.
(b) Name one impurity which is removed at purification stage.
(c) Label on the diagram each of the following:
Anode
Cathode
Region containing the electrolyte
(d) The melting point of aluminium oxide is 20540C but electrolysis is done between 8000C -
9000C.
(i) Why is the electrolysis not carried out at 20540C.?
(ii) What is done to lower the temperature of the electrolysis cell to 8000C - 9000C?
(iii) The aluminium which is produced is tapped off as liquid. What does this imply
about its melting point?
(e) A typical electrolysis cell uses a current of 40000 ampheres. Calculate the mass (in kilograms) of aluminium produced in one hour.
Date posted:
May 20, 2019
.
Answers (1)
-
The flow chart below outlines some of the process involved during extraction of copper.
(Solved)
The flow chart below outlines some of the process involved during extraction of copper.

a) (i) Write the formula of copper pyrite.
(ii) Name liquid T
(iii) Write equations for the reactions taking place in the 2nd roasting furnace.
(iv) Identify substance B and write equation for the reaction that take place in the smelting furnace.
(v) State the purpose of substance F.
Date posted:
May 20, 2019
.
Answers (1)
-
Study the diagram below and answer the questions which follow.
(Solved)
Study the diagram below and answer the questions which follow.
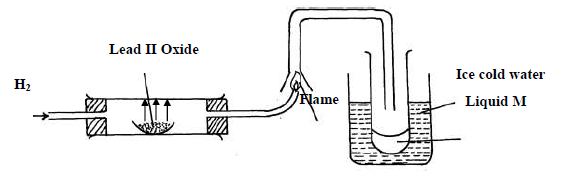
(i) State two observations made when hydrogen gas pass over hot lead II oxide.
(ii) Write the equation for the reaction which occurs in the combustion tube.
(iii) What property of hydrogen is shown in the experiment above
(iv) Identify liquid M.
(v) What type of reaction occurs when hydrogen gas reacts with butene?
(vi) State the condition required for the reaction (v) above
(vii) Apart from hydrogen peroxide, state two other reagents that can be used to prepare
oxygen gas.
(viii) Write an equation to show how hydrogen gas is formed from the reagents chosen in
(vii) above.
Date posted:
May 17, 2019
.
Answers (1)
-
Give an equation to show how chlorine forms bleaching powder.
(Solved)
Give an equation to show how chlorine forms bleaching powder.
Date posted:
May 17, 2019
.
Answers (1)
-
The set up below was used to prepare chlorine gas. (i) Identify solid M (ii) What is the role of water in the experiment?.....
(Solved)
The set up below was used to prepare chlorine gas.

(i) Identify solid M
(ii) What is the role of water in the experiment?
(iii) Complete the set up to show how dry chlorine gas can be collected
(iv) Write a chemical equation to show how chlorine gas is formed.
Date posted:
May 17, 2019
.
Answers (1)
-
The flow chart below summarizes the process of extraction of lead from a chief ore.
(Solved)
The flow chart below summarizes the process of extraction of lead from a chief ore.

(i) Identify process T
(ii) Give the name of:
Gas N
Solid F
(iii) Give two functions of CaCO3 in the extraction process.
(iv) Write an equation to show how waste product J is formed.
Date posted:
May 17, 2019
.
Answers (1)
-
A sealed glass tube containing 250 cm3 of nitrogen gas at r.t.p was immersed in boiling water. Calculate the pressure inside the tube if the...
(Solved)
A sealed glass tube containing 250 cm3 of nitrogen gas at r.t.p was immersed in boiling water. Calculate the pressure inside the tube if the volume of the gas does not change due to expansion of glass. (Room pressure=760mmHg, room temperature=298K).
Date posted:
May 17, 2019
.
Answers (1)
-
A solution of bromine in methyl benzene turns colourless when butane gas is passed through it.
(Solved)
A solution of bromine in methyl benzene turns colourless when butane gas is passed through it.
(a) What type of reaction takes place?
(b) Write equation of the reaction which takes place.
Date posted:
May 17, 2019
.
Answers (1)
-
60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide...
(Solved)
60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide (NO2) to diffuse under the same conditions. (O=16,N=14).
Date posted:
May 17, 2019
.
Answers (1)
-
The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that...
(Solved)
The diagram below is a set up used to investigate the effect of heat on hydrated copper(II)
sulphate. Study the diagram and answer the questions that follow.
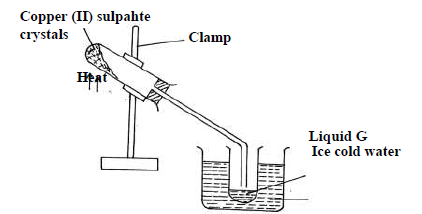
(a) Why is boiling tube slanted as shown?
(b) What is observed in the boiling tube.
(c) Identify liquid G.
Date posted:
May 17, 2019
.
Answers (1)
-
Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.
(Solved)
Explain why the following combination of reagents is unsuitable for the laboratory
preparation of hydrogen.
(i) Zinc + dilute nitric acid.
(ii) Lead + dilute hydrochloric acid.
(iii) Potassium + dilute sulphuric acid.
Date posted:
May 17, 2019
.
Answers (1)