(a) U, T, S , R, Q, P in that order.
Increasing atomic radius.
(b) Both are metals/both react by losing electrons.
sharon kalunda answered the question on May 20, 2019 at 12:14
- A mixture contains sodium chloride, ammonium chloride, and silver chloride. Explain how
you can obtain pure samples of each salt.(Solved)
A mixture contains sodium chloride, ammonium chloride, and silver chloride. Explain how
you can obtain pure samples of each salt.
Date posted: May 20, 2019. Answers (1)
- Use the diagram below and answer the questions that follow.
The above experiment was performed using carbon electrode and another electrode
(i) Identify electrode B
(ii) Name...(Solved)
Use the diagram below and answer the questions that follow.
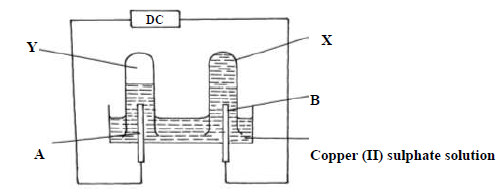
The above experiment was performed using carbon electrode and another electrode
(i) Identify electrode B
(ii) Name the colourless gas observed in test tube Y
(iii) Explain why no gas was observed in list tube X
Date posted: May 20, 2019. Answers (1)
- The solubility of salt x at various temperature is as shown in the data given below.(Solved)
The solubility of salt x at various temperature is as shown in the data given below.
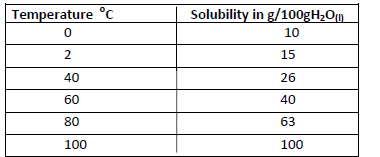
Using a suitable scale draw a solubility curve of salt x on the grid provided below
Date posted: May 20, 2019. Answers (1)
- State and explain the observations made when sodium metal is put into a boiling tube containing propan–l-ol(Solved)
State and explain the observations made when sodium metal is put into a boiling tube containing propan–l-ol
Date posted: May 20, 2019. Answers (1)
- State two industrial use of methane.(Solved)
State two industrial use of methane.
Date posted: May 20, 2019. Answers (1)
- (i) Write an equation for the reaction between propan – l- ol and sodium metal.
(ii) Name process I and II
(iii) Identify the products A...(Solved)

(i) Write an equation for the reaction between propan – l- ol and sodium metal.
(ii) Name process I and II
(iii) Identify the products A and B
(iv) Name catalyst used in product II
(v) Draw the structural formula of the repeating unit to the polymer C
Date posted: May 20, 2019. Answers (1)
- Raw rubber is heated with sulphur in the manufacture of natural rubber.(Solved)
Raw rubber is heated with sulphur in the manufacture of natural rubber.
(i) What name is given to the process?
(ii Why is the process necessary.
Date posted: May 20, 2019. Answers (1)
- To which homologous series do the following compounds belong?(Solved)
(a)To which homologous series do the following compounds belong?

(b) Draw and name the isomers of butyne
Date posted: May 20, 2019. Answers (1)
- State four industrial uses of Aluminium(Solved)
State four industrial uses of Aluminium
Date posted: May 20, 2019. Answers (1)
- The diagram below shows method used to extract aluminium by the electrolysis of molten
bauxite.
(i) Give equation for the reaction occurring at the two electrode.
Anode
Cathode...(Solved)
The diagram below shows method used to extract aluminium by the electrolysis of molten
bauxite.
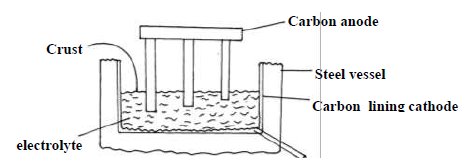
(i) Give equation for the reaction occurring at the two electrode.
Anode
Cathode
(ii) In this process the anode rod have to be replaced from time to time. Explain.
(iii) The working temperature in this cell is below the normal melting point of the
purified ore. Explain the significance of this situation and how it is achieved.
Date posted: May 20, 2019. Answers (1)
- The table below gives some properties of three metals: Aluminium, iron and copper. Use it to
answer the questions that follow.(Solved)
The table below gives some properties of three metals: Aluminium, iron and copper. Use it to
answer the questions that follow.

Assuming that steel and stainless steel have similar properties to iron.
(a) Why do some stainless steel sauce pans have a copper base?
(b) Aluminum with a steel core is used for overhead power cables in preference to
copper. Why is aluminum preferred ?
( c) Apart from over head power cables copper is chosen for almost all other electrical uses. Suggest two reasons fort he choice of copper.
Date posted: May 20, 2019. Answers (1)
- Iron has two oxidation states, so it can form ions Fe2+. How can you test a solution to find out which ion is present. Outline...(Solved)
Iron has two oxidation states, so it can form ions Fe2+. How can you test a solution to find out which ion is present. Outline the tests and give the results for both ions.
Date posted: May 20, 2019. Answers (1)
- The diagram below shows two types of detergents which one of these detergents is a soap?
Give a reason for your choice.(Solved)
The diagram below shows two types of detergents which one of these detergents is a soap?
Give a reason for your choice.
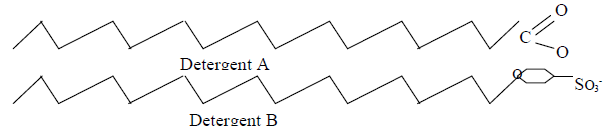
Date posted: May 20, 2019. Answers (1)
- Below is a table of first five alkanes and their boiling points.(Solved)
Below is a table of first five alkanes and their boiling points.

What is the state of pentane at room temperature ( 25oC)? Give a reasons.
Date posted: May 20, 2019. Answers (1)
- Hydrogen peroxide decomposes according to the equation shown below.(Solved)
Hydrogen peroxide decomposes according to the equation shown below.
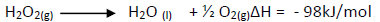
8.5g of hydrogen peroxide contained in 100cm3 of solution with water were completely
decomposed.Calculate the rise in temperature due to the reaction.(specific heat capacity on water = 4.25
Date posted: May 20, 2019. Answers (1)
- In a closed system an equilibrium exists between nitrogen(IV) oxide and dinitrogen tetraoxide
as shown in the equation.(Solved)
In a closed system an equilibrium exists between nitrogen(IV) oxide and dinitrogen tetraoxide
as shown in the equation.
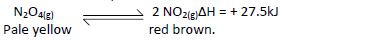
State and explain the observation made when a glass syringe containing the equilibrium mixture is immersed in ice-cold water.
Date posted: May 20, 2019. Answers (1)
- A concentrated solution of sulphuric (VI) acid contain 72.5% sulphuric (VI) acid. If the density
of the acid is 1.8g/cm3 determine the molarity of the acid...(Solved)
A concentrated solution of sulphuric (VI) acid contain 72.5% sulphuric (VI) acid. If the density
of the acid is 1.8g/cm3 determine the molarity of the acid solution. (H= 1, O=16, S = 32)
Date posted: May 20, 2019. Answers (1)
- What is the colour of the following?(Solved)
What is the colour of the following?


Date posted: May 20, 2019. Answers (1)
- Chlorine reacts with methane as shown below.
(a) What condition is necessary for this reaction to take place?
(b) Identify the bonds which are broken and those...(Solved)
Chlorine reacts with methane as shown below.
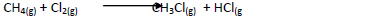
(a) What condition is necessary for this reaction to take place?
(b) Identify the bonds which are broken and those that are formed.
(i) Bonds broken.
(ii) Bonds formed.
Date posted: May 20, 2019. Answers (1)
- Hydrogen and Flourine react according to the equation. On the grid provided below, sketch the energy level diagram for the reverse reaction.(Solved)
Hydrogen and Flourine react according to the equation.
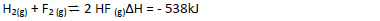
On the grid provided below, sketch the energy level diagram for the reverse reaction.
(b) Calculate the molar enthalpy of formation of HF.
Date posted: May 20, 2019. Answers (1)