- State one large scale use of sulphuric (VI) acid(Solved)
State one large scale use of sulphuric (VI) acid
Date posted: May 20, 2019. Answers (1)
- Sulphuric acid is manufactured in large scale by the contact process. The basic reaction in the
contact process is catalytic oxidation of sulphur(IV) oxide.(Solved)
Sulphuric acid is manufactured in large scale by the contact process. The basic reaction in the
contact process is catalytic oxidation of sulphur(IV) oxide.
(a) Name the catalyst used.
(b) Write an equation for the basic reaction.
Date posted: May 20, 2019. Answers (1)
- Nelly’s lungs can hold 2500cm3 of air at 37oC and 1 atmosphere. What would be the pressure if this air was put in a bottle...(Solved)
Nelly’s lungs can hold 2500cm3 of air at 37oC and 1 atmosphere. What would be the pressure if this air was put in a bottle of capacity 500cm3 at 27oC?
Date posted: May 20, 2019. Answers (1)
- Explain why galvanized iron objects are better protected even when scratched.(Solved)
Explain why galvanized iron objects are better protected even when scratched.
Date posted: May 20, 2019. Answers (1)
- Painting, oiling, galvanizing or tin-plating are methods of preventing rusting.Explain how these methods are similar in the way they prevent rusting.(Solved)
Painting, oiling, galvanizing or tin-plating are methods of preventing rusting.Explain how these methods are similar in the way they prevent rusting.
Date posted: May 20, 2019. Answers (1)
- The following set up was used to react steam with Iron Powder.
(a) The water was heated before heating the iron powder. Explain why this was...(Solved)
The following set up was used to react steam with Iron Powder.

(a) The water was heated before heating the iron powder. Explain why this was necessary.
(b) Write an equation for the reaction that took place between steam and iron powder
(c) State how gas L would be collected without using water.
Date posted: May 20, 2019. Answers (1)
- 25cm3 of 0.1m sulphuric (VI) acid required 20cm3 of sodium carbonate solution for complete neutralization. Calculate the concentration of sodium carbonate in moles per litre.(Solved)
25cm3 of 0.1m sulphuric (VI) acid required 20cm3 of sodium carbonate solution for complete neutralization. Calculate the concentration of sodium carbonate in moles per litre.
Date posted: May 20, 2019. Answers (1)
- (a) Identify the particles emitted at step I and Step II
(b) Write the nuclear equation for the reaction which takes place in step (II)
(c)...(Solved)

(a) Identify the particles emitted at step I and Step II
(b) Write the nuclear equation for the reaction which takes place in step (II)
Date posted: May 20, 2019. Answers (1)
- The graph below represents the solubility curve of a gas in water.
(a) State and explain the conclusion that can be drawn from this curve about...(Solved)
The graph below represents the solubility curve of a gas in water.

(a) State and explain the conclusion that can be drawn from this curve about the
solubility of the gas.
(b) The solubility of potassium chlorate at 80oC is 40g/100g of water. What mass of potassium chlorate will saturate 65g of water at 80oC.
Date posted: May 20, 2019. Answers (1)
- Iron is extracted from its ore, hematite in the blast furnace. The main reaction during
extraction is shown by the equation below;
Calculate the mass of iron...(Solved)
Iron is extracted from its ore, hematite in the blast furnace. The main reaction during
extraction is

Calculate the mass of iron which will be produced from 320 tonnes of hematite.
(Fe= 56 O=16)
Date posted: May 20, 2019. Answers (1)
- 6.5 g of zinc granules were reacted with 25cm3 of 4M hydrochloric acid. The graph below shows
the results:
(a) How long did it take for...(Solved)
6.5 g of zinc granules were reacted with 25cm3 of 4M hydrochloric acid. The graph below shows
the results:

(a) How long did it take for the reaction to be complete?
(b) Calculate the average rate of reaction.
Date posted: May 20, 2019. Answers (1)
- Flourine has very low melting and boiling points and yet its atoms are joined by covalent bonding. Explain.(Solved)
Fluorine has very low melting and boiling points and yet its atoms are joined by covalent
bonding. Explain.
Date posted: May 20, 2019. Answers (1)
- Using dots (•) and cross (x) show the bonding in hydroxonium ion(Solved)
Using dots (•) and cross (x) show the bonding in hydroxonium ion
Date posted: May 20, 2019. Answers (1)
- A compound W react with chlorine to form another compound Y whose structural
formula is as follows:
(i) Give the name and structural formula of Compound W...(Solved)
A compound W react with chlorine to form another compound Y whose structural
formula is as follows:

(i) Give the name and structural formula of Compound W
(ii) What type of reaction leads to the formation of compound Y from compound W.
Date posted: May 20, 2019. Answers (1)
- Draw the structure of the following compounds:
(i) 2 – Methyprop-1-ene
(ii) Hexan – 2- ol(Solved)
Draw the structure of the following compounds:
(i) 2 – Methyprop-1-ene
(ii) Hexan – 2- ol
Date posted: May 20, 2019. Answers (1)
- Study the reaction scheme below and answer the questions that follow.
(a) Suggest the possible anions in Y and V
(b) Predict the name of gas...(Solved)
Study the reaction scheme below and answer the questions that follow.
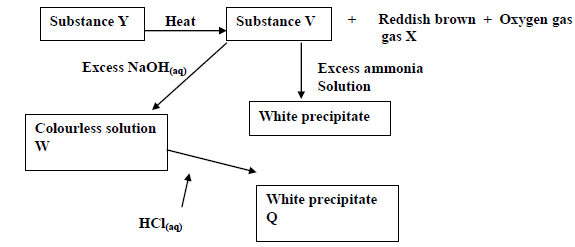
(a) Suggest the possible anions in Y.
(b) Predict the name of gas X.
Date posted: May 20, 2019. Answers (1)
- Elements Q,S,T,U,R and P belong to the same period in the periodic table. The ions formed
by the atoms of the elements are given below: Q2+,...(Solved)
Elements Q,S,T,U,R and P belong to the same period in the periodic table. The ions formed
by the atoms of the elements are given below: Q2+, U- , T2-, R3+, P+ and S3- .
(a) Arrange the elements in order of increasing atomic size.
(b) Suggest a reason why elements P and Q cannot react with each other to form a
compound.
Date posted: May 20, 2019. Answers (1)
- A mixture contains sodium chloride, ammonium chloride, and silver chloride. Explain how
you can obtain pure samples of each salt.(Solved)
A mixture contains sodium chloride, ammonium chloride, and silver chloride. Explain how
you can obtain pure samples of each salt.
Date posted: May 20, 2019. Answers (1)
- Use the diagram below and answer the questions that follow.
The above experiment was performed using carbon electrode and another electrode
(i) Identify electrode B
(ii) Name...(Solved)
Use the diagram below and answer the questions that follow.
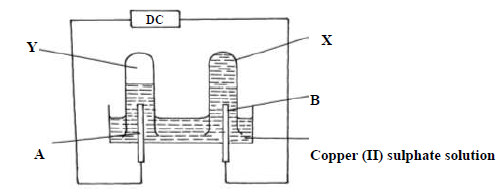
The above experiment was performed using carbon electrode and another electrode
(i) Identify electrode B
(ii) Name the colourless gas observed in test tube Y
(iii) Explain why no gas was observed in list tube X
Date posted: May 20, 2019. Answers (1)
- The solubility of salt x at various temperature is as shown in the data given below.(Solved)
The solubility of salt x at various temperature is as shown in the data given below.
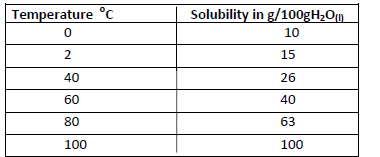
Using a suitable scale draw a solubility curve of salt x on the grid provided below
Date posted: May 20, 2019. Answers (1)