- The fig below shows a hydraulic press used to compress a bale
(i) Explain briefly ho a force applied on the lever compresses the bale.
(ii) Given...(Solved)
The fig below shows a hydraulic press used to compress a bale
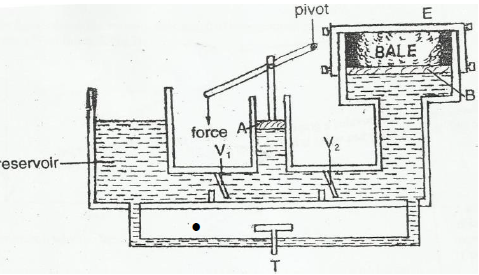
(i) Explain briefly ho a force applied on the lever compresses the bale.
(ii) Given that the area of piston B is 18cm2 and that of piston A 3.0cm3. A force of 2N is
applied to piston A, find the force produced on the larger piston B that compresses
the bale.
Date posted: May 21, 2019. Answers (1)
- When a fountain pen is taken in a high aeroplane, it leaks. A ball point pen does not have this problem. Explain how the ball...(Solved)
When a fountain pen is taken in a high aero plane, it leaks. A ball point pen does not have this
problem. Explain how the ball point is able to overcome this problem.
Date posted: May 21, 2019. Answers (1)
- Sketch on he axes provided a graph to show how mass per unit volume of water varies with
temperature when water is heated from 00 to...(Solved)
Sketch on he axes provided a graph to show how mass per unit volume of water varies with
temperature when water is heated from 00 to 200
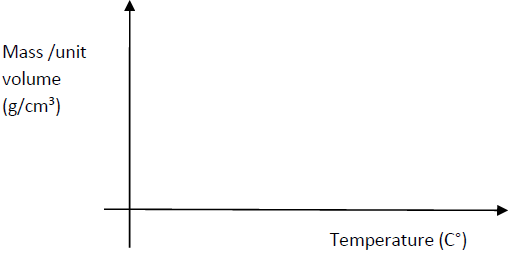
Date posted: May 21, 2019. Answers (1)
- On the axes provided sketch a graph of velocity (v) verses time (t) for uniformly accelerated
motion given that t=0,v is greater than zero.(Solved)
On the axes provided sketch a graph of velocity (v) verses time (t) for uniformly accelerated
motion given that t=0,v is greater than zero.
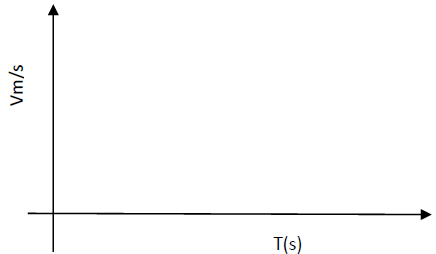
Date posted: May 21, 2019. Answers (1)
- A box of mass 500g is dragged along a level ground at a speed of 12m/s. if the force of friction
between the box and the...(Solved)
A box of mass 500g is dragged along a level ground at a speed of 12m/s. if the force of friction
between the box and the floor is 2000N, calculate the power developed.
Date posted: May 21, 2019. Answers (1)
- The figure shows the velocity time graph of two identical spheres released from the surfaces of
two liquids A and B.
Give a reason why the terminal...(Solved)
The figure shows the velocity time graph of two identical spheres released from the surfaces of
two liquids A and B.
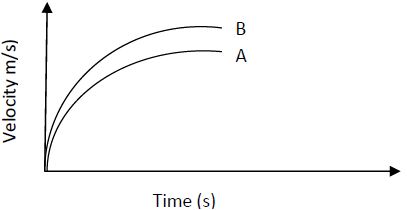
Give a reason why the terminal velocity of the sphere In B is higher than in A.
Date posted: May 21, 2019. Answers (1)
- When a Bunsen burner is lit below wire gauze, it is noted the flame initially burns below the gauze as shown in the diagram below. After sometime, the flame burns...(Solved)
When a Bunsen burner is lit below wire gauze, it is noted the flame initially burns below the gauze
as shown in the figure below.
After sometime, the flame burns below as well as above the gauze as shown in
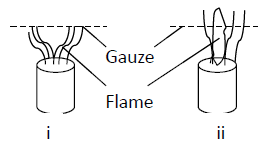
Explain this observation
Date posted: May 21, 2019. Answers (1)
- The figure below shows a uniform bar pivoted at its centre and is at equilibrium.
Determine the value of w.(Solved)
The figure below shows a uniform bar pivoted at its centre and is at equilibrium.
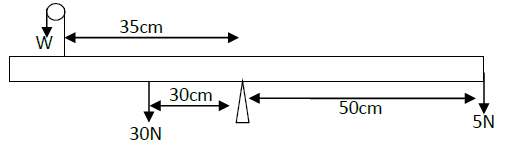
Determine the value of w.
Date posted: May 21, 2019. Answers (1)
- An immersion heater rated at 180W is placed in a liquid of mass 2kg. When the heater is switched on for 7.5 minutes the temperature...(Solved)
An immersion heater rated at 180W is placed in a liquid of mass 2kg. When the heater is switched on for 7.5 minutes the temperature of the liquid rises by 400C. Determine the specific heat capacity of the liquid.
Date posted: May 21, 2019. Answers (1)
- Other than oil patch being monolayer, state any one other assumption in the oil drop experiment.(Solved)
Other than oil patch being monolayer, state any one other assumption in the oil drop experiment.
Date posted: May 21, 2019. Answers (1)
- Estimate the size of an oil molecule if a drop of oil of volume 6.0 × 10-10 m3 forms a patch of 32 on a water...(Solved)
Estimate the size of an oil molecule if a drop of oil of volume 6.0 × 10-10 m3 forms a patch of 32 on a water surface.
Date posted: May 21, 2019. Answers (1)
- The following figure shows a rod made of wood on one end and metal on the other end
suspended freely with a piece of thread so...(Solved)
The following figure shows a rod made of wood on one end and metal on the other end
suspended freely with a piece of thread so that it is in equilibrium.
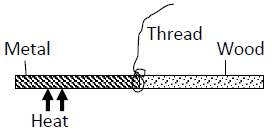
The side made of metal is now heated with a Bunsen flame. State with a reason, the side to
which the rod is likely to tilt
Date posted: May 21, 2019. Answers (1)
- A stone of mass 40g was completely immersed in a liquid. The level of liquid are shown in the figure. Determine the density of the stone in...(Solved)
A stone of mass 40g was completely immersed in a liquid. The level of liquid are shown in the figure
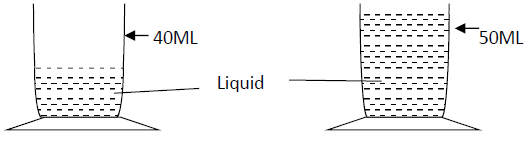
Determine the density of the stone in SI units
Date posted: May 21, 2019. Answers (1)
- In the clothing and textile industries the machines experience electrostatic forces at certain points. Suggest one method of reducing these forces(Solved)
In the clothing and textile industries the machines experience electrostatic forces at certain points. Suggest one method of reducing these forces
Date posted: May 21, 2019. Answers (1)
- A wire was connected to a battery and was found that the energy converted to heat was 30J when 20C of charge flowed through the...(Solved)
A wire was connected to a battery and was found that the energy converted to heat was 30J when 20C of charge flowed through the wire in 5 seconds. Calculate;
i) The p.d between the ends of the wire
ii) The current flowing through the wire
iii)The resistance of the wire
iv)The average power development in the wire
Date posted: May 20, 2019. Answers (1)
- Sketch a simple diagram that contains a capacitor, a two way switch, and a load resistor that can be used for charging and discharging a...(Solved)
Sketch a simple diagram that contains a capacitor, a two way switch, and a load resistor that can be used for charging and discharging a capacitor.
Date posted: May 20, 2019. Answers (1)
- The figure below shows three capacitors connected between two points A and B.
Determine the capacitance across AB(Solved)
The figure below shows three capacitors connected between two points A and B.

Determine the capacitance across AB
Date posted: May 20, 2019. Answers (1)
- Figure below shows a ray of light incident on the face of a cube made of glass refractive index 1.50. Calculate (i) The angle r: (ii) The critical...(Solved)
Figure below shows a ray of light incident on the face of a cube made of glass refractive index 1.50
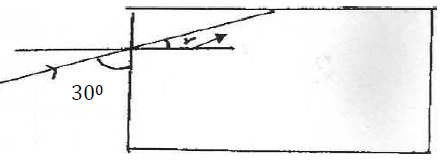
Calculate
i) The angle r:
ii) The critical angle for the glass air interface
Date posted: May 20, 2019. Answers (1)
- A gun is fired and an echo heard at the same place 0.6s later. How far is the barrier, which reflected the sound from the...(Solved)
A gun is fired and an echo heard at the same place 0.6s later. How far is the barrier, which reflected the sound from the gun? (Speed of sound in air=330ms-1
Date posted: May 20, 2019. Answers (1)
- Figure below shows two plane mirrors inclined at an angle x from each other. A viewer counts a total of seven images by looking directly...(Solved)
Figure below shows two plane mirrors inclined at an angle x from each other. A viewer counts a total of seven images by looking directly from the object O. Determine value of angel x.
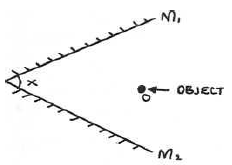
Date posted: May 20, 2019. Answers (1)