- Use the information below on standard electrode potentials to answer the questions that
follow:(Solved)
Use the information below on standard electrode potentials to answer the questions that
follow:
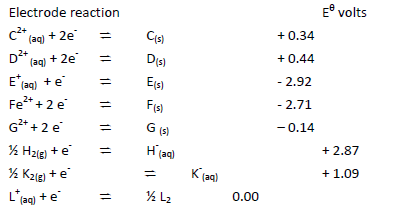
a) (i) Identify the strongest reducing agent and the strongest oxidizing agent. Give reasons.
(ii) Calculate the e.m.f of the cell formed by connecting half cells C and D.
b) Draw and label a diagram of a cell formed by –connecting half cells of E and D. On the
diagram indicate the flow of electrons.
Date posted: May 21, 2019. Answers (1)
- A compound of carbon, hydrogen and oxygen contains 71.12 by mass of oxygen, 2.2 hydrogen and the rest is carbon. It has relative molecular mass...(Solved)
A compound of carbon, hydrogen and oxygen contains 71.12 by mass of oxygen, 2.2 hydrogen and the rest is carbon. It has relative molecular mass of 90.Determine the empirical formula of the compound.
Date posted: May 21, 2019. Answers (1)
- Study the information below and answer the following questions. A mixture contains three
solid A,B and C. the solubility of these solids in different liquids is...(Solved)
Study the information below and answer the following questions. A mixture contains three
solid A,B and C. the solubility of these solids in different liquids is as shown below
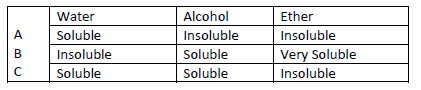
Explain how you will obtain sample C from the mixture.
Date posted: May 21, 2019. Answers (1)
- The chemical equations below are the main reactions in large scale manufacture of sodium carbonate.
a) Explain how the two products, NaHCO3 and NH4Cl are separated....(Solved)
The chemical equations below are the main reactions in large scale manufacture of sodium carbonate.

a) Explain how the two products, NaHCO3 and NH4Cl are separated.
b)How is sodium carbonate finally obtained?
c) Explain how ammonia is recovered and recycled?
Date posted: May 21, 2019. Answers (1)
- The peaks below show the mass spectrum of element X.
Calculate the relative atomic mass of X.(Solved)
The peaks below show the mass spectrum of element X.

Calculate the relative atomic mass of X.
Date posted: May 21, 2019. Answers (1)
- State two uses of Argon.(Solved)
State two uses of Argon.
Date posted: May 21, 2019. Answers (1)
- Solutions can be classified as acids bases or neutral. The table below shows solutions and their pH values.(Solved)
Solutions can be classified as acids bases or neutral. The table below shows solutions and their pH values.

(i) Select any pair that would react to form a solution of pH 7
(ii) Identify two solutions that would react with Aluminium hydroxide. Explain.
Date posted: May 21, 2019. Answers (1)
- Study the following equilibrium reaction. Suggest two ways of increasing the yield of A2B.(Solved)
Study the following equilibrium reaction
 .
.
Suggest two ways of increasing the yield of A2B.
Date posted: May 21, 2019. Answers (1)
- D grams of potassium hydroxide were dissolved in distilled water to make 100cm3 of
solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for...(Solved)
D grams of potassium hydroxide were dissolved in distilled water to make 100cm3 of
solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for complete neutralization.Calculate the mass D of potassium hydroxide.

(relative formula of KOH=56)
Date posted: May 21, 2019. Answers (1)
- Element A has atomic mass 23 and element B atomic mass 7 and also have 12 neutrons and 4 neutrons respectively.(Solved)
Element A has atomic mass 23 and element B atomic mass 7 and also have 12 neutrons and 4 neutrons respectively.
a) Write the electron arrangement of A and B
b) Which element has higher ionization energy? Explain
Date posted: May 21, 2019. Answers (1)
- When hydrogen chloride gas is dissolved in water the solution formed turns blue litmus paper red but there is no effect on blue litmus paper...(Solved)
When hydrogen chloride gas is dissolved in water the solution formed turns blue litmus paper red but there is no effect on blue litmus paper when the gas is dissolved in carbon tetra chloride.Explain
Date posted: May 21, 2019. Answers (1)
- When lead (II) carbonate reacts with dilute hydrochloric acid, very little carbon (iv) oxide is produced.Explain(Solved)
When lead (II) carbonate reacts with dilute hydrochloric acid, very little carbon (iv) oxide is produced.Explain
Date posted: May 21, 2019. Answers (1)
- Using reagents provided only, explain by means of balanced chemical equations how you
could prepare a salt of Zinc carbonate solid.
Zinc powder
Nitric (V) acid...(Solved)
Using reagents provided only, explain by means of balanced chemical equations how you
could prepare a salt of Zinc carbonate solid.
Zinc powder
Nitric (V) acid (dilute)
Water
Solid sodium carbonate
Date posted: May 21, 2019. Answers (1)
- Consider the Zinc nuclide below
Determine the number of protons and neutrons in the nuclide.(Solved)
Consider the Zinc nuclide below

Determine the number of protons and neutrons in the nuclide.
Date posted: May 21, 2019. Answers (1)
- Given the equation for reaction below Calculate
(i) Volume of chlorine at (r.t.p) required to react with 3g of Aluminium (Molar gas volume at r.t.p =...(Solved)
Given the equation for reaction below

Calculate
(i) Volume of chlorine at (r.t.p) required to react with 3g of Aluminium (Molar gas volume at r.t.p = 24litres, Al = 27, Cl = 35.5).
(ii) Mass of Aluminium chloride formed.
Date posted: May 21, 2019. Answers (1)
- Calculate the solubility of sugar in water at 40oC from the following information.
Mass of evaporating dish = 23.0g
Mass of evaporating dish + sample of saturated...(Solved)
Calculate the solubility of sugar in water at 40oC from the following information.
Mass of evaporating dish = 23.0g
Mass of evaporating dish + sample of saturated solution = 192.0g
Mass of evaporation dish + solid after evaporating of solution + 142.0g
Date posted: May 21, 2019. Answers (1)
- Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions.(Solved)
Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions.
Date posted: May 21, 2019. Answers (1)
- The apparatus below was a set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow.
(i) Write an...(Solved)
The apparatus below was a set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow.

(i) Write an equation for the reaction that takes place in the gas jar.
(ii) Why is it necessary to have a hot nichrome wire in the gas jar.
Date posted: May 21, 2019. Answers (1)
- Use the chart below to answer the questions that follow.
Identify:
Gas P
Solid R
Solid T .
Liquid S(Solved)
Use the chart below to answer the questions that follow.

Identify:
Gas P
Solid R
Solid T .
Liquid S
Date posted: May 21, 2019. Answers (1)
- Name the following compounds using the IUPAC system.(Solved)
Name the following compounds using the IUPAC system.

Date posted: May 21, 2019. Answers (1)