(i)The roasted ore is heated with concentrated. Sodium hydroxide under pressure, Aluminium oxide being amphoteric dissolves in Sodium hydroxide.
(ii) Iron (III) oxide/ Iron hydroxide does not dissolve (insoluble in NaOH)
(iii) Cryolite lowers the melting point of alumina.
sharon kalunda answered the question on May 21, 2019 at 09:02
- Describe how sulphuric acid is manufactured from sulphur (VI) oxide.(Solved)
Describe how sulphuric acid is manufactured from sulphur (VI) oxide.
Date posted: May 21, 2019. Answers (1)
- By using a chemical test, how can you distinguish H2S(g) and SO2(g).(Solved)
By using a chemical test, how can you distinguish H2S(g) and SO2(g).
Date posted: May 21, 2019. Answers (1)
- Study the diagram below and answer the questions that follow
(i) Identify the reagents
(ii) Name the yellow solid.(Solved)
Study the diagram below and answer the questions that follow
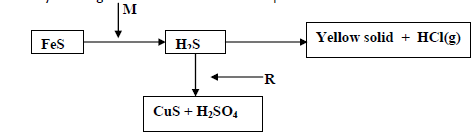
a)(i) Identify the reagents M and R
(ii) Name the yellow solid.
b) What would be the effect of the yield of sulphur (VI) oxide when
(i) Increasing the concentration of oxygen.
(ii) Increasing the temperature .
Date posted: May 21, 2019. Answers (1)
- The diagram below shows the preparation of nitric acid.
a) Name solid A.
b) Under what conditions does sulphuric acid react with solid A.
c) What is the...(Solved)
The diagram below shows the preparation of nitric acid.
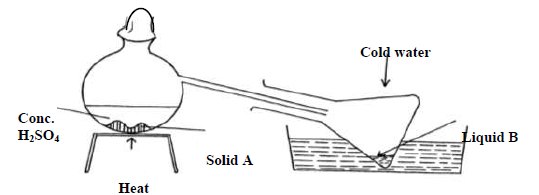
a) Name solid A.
b) Under what conditions does sulphuric acid react with solid A.
c) What is the colour of liquid B
d) What is the purpose of cold water
e) 1 cm3 of liquid B was diluted with distilled water and a few drops of copper turnings dropped into it A colourless gas and later brown gas were produced.
(i) Name the colourless gas
(ii) Name the brown gas formed?
(iii) Give an equation for the formation of the brown gas
Date posted: May 21, 2019. Answers (1)
- Elements V,W and X have atomic number 17,19 and 20 respectively.
(a) What is the valencies of V and W respectively.
(b) To which groups of the...(Solved)
Elements V,W and X have atomic number 17,19 and 20 respectively.
(a) What is the valencies of V and W respectively.
(b) To which groups of the periodic table do V, and X belong.
(c) In which periods do elements V and W lie.?
(d) Which of the three elements is a non-metal?
(e) Write down the formula of the compounds formed when:
(i) V reacts with W
(ii) X reacts with Oxygen
f) How many
(i) Neutrons does V have? if its mass number is 35
(ii) Protons does W have ?
Date posted: May 21, 2019. Answers (1)
- In the preparation of magnesium carbonate magnesium was burnt in air and the product collected.Dilute sulphuric acid was added and the mixture filtered and cooled....(Solved)
In the preparation of magnesium carbonate magnesium was burnt in air and the product collected.Dilute sulphuric acid was added and the mixture filtered and cooled. Sodium carbonate was added to the filtrate and the content filtered. The residue was washed and dried to give a white powder.
a) Give the chemical name of the product formed when magnesium burns in air
b) Write a chemical equation for the formation of product.
c) (i) Name filtrate collected after sodium carbonate was added
(ii) Name the white powder.
d) Write chemical equation for the reaction between product in (a) and acid.
e) Name the ions present in the filtrate after addition of sodium carbonate.
f) Write an ionic equation to show the formation of the white powder
g) Write an equation to show what happened when white powder is strongly heated.
Date posted: May 21, 2019. Answers (1)
- The scheme below shows some products that can be obtained starting from ethene.
(i) Name the compounds
(ii) Name the process
(iii) State one condition necessary...(Solved)
The scheme below shows some products that can be obtained starting from ethene.
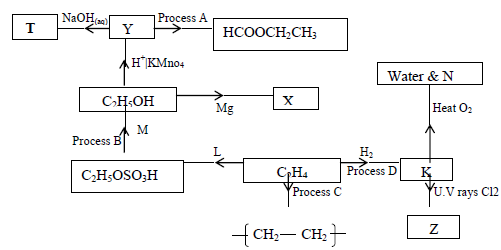
(i) Name the compounds M,N,T,X,Y and Z
(ii) Name the process B,C and D
(iii) State one condition necessary for the processes in (ii) above to take place.
Date posted: May 21, 2019. Answers (1)
- Filter paper dipped in acidified Potassium Manganate (VII) were placed in two
separate gas jars A and B containing pentane and Pent-l-ene respectively. Explain
what was observed...(Solved)
Filter paper dipped in acidified Potassium Manganate (VII) were placed in two
separate gas jars A and B containing pentane and Pent-l-ene respectively. Explain
what was observed in each case.
Date posted: May 21, 2019. Answers (1)
- Draw one of the positional isomer of Butene.(Solved)
Draw one of the positional isomer of Butene.
Date posted: May 21, 2019. Answers (1)
- An aqueous solution of Copper (II) Sulphate was electrolysed using platinum electrodes. When a current was passed a gas that relights a glowing splint was...(Solved)
An aqueous solution of Copper (II) Sulphate was electrolysed using platinum electrodes. When a current was passed a gas that relights a glowing splint was produced.
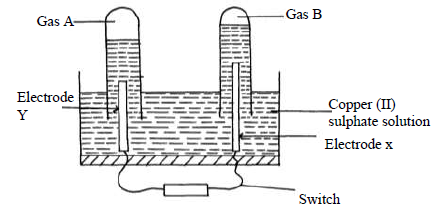
(i) Name the electrode which acts as cathode. Give a reason.
(ii) Write an equation for the reaction at the anode.
d) 0.11g of metal R deposited by electrolysis when a current of 0.03 amperes flow for
99 minutes. ( R =92.) ,(1 Faraday = 96500 C)
(i) Find the number of moles of metal deposited.
(ii) Find the number of moles of electrons passed.
(iii) Determine the value of n in the metallic ion Rn+.
Date posted: May 21, 2019. Answers (1)
- Use the information below on standard electrode potentials to answer the questions that
follow:(Solved)
Use the information below on standard electrode potentials to answer the questions that
follow:
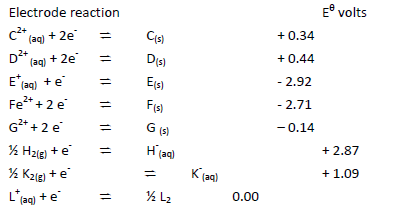
a) (i) Identify the strongest reducing agent and the strongest oxidizing agent. Give reasons.
(ii) Calculate the e.m.f of the cell formed by connecting half cells C and D.
b) Draw and label a diagram of a cell formed by –connecting half cells of E and D. On the
diagram indicate the flow of electrons.
Date posted: May 21, 2019. Answers (1)
- A compound of carbon, hydrogen and oxygen contains 71.12 by mass of oxygen, 2.2 hydrogen and the rest is carbon. It has relative molecular mass...(Solved)
A compound of carbon, hydrogen and oxygen contains 71.12 by mass of oxygen, 2.2 hydrogen and the rest is carbon. It has relative molecular mass of 90.Determine the empirical formula of the compound.
Date posted: May 21, 2019. Answers (1)
- Study the information below and answer the following questions. A mixture contains three
solid A,B and C. the solubility of these solids in different liquids is...(Solved)
Study the information below and answer the following questions. A mixture contains three
solid A,B and C. the solubility of these solids in different liquids is as shown below
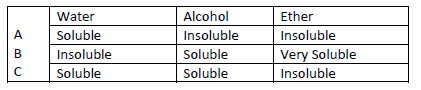
Explain how you will obtain sample C from the mixture.
Date posted: May 21, 2019. Answers (1)
- The chemical equations below are the main reactions in large scale manufacture of sodium carbonate.
a) Explain how the two products, NaHCO3 and NH4Cl are separated....(Solved)
The chemical equations below are the main reactions in large scale manufacture of sodium carbonate.

a) Explain how the two products, NaHCO3 and NH4Cl are separated.
b)How is sodium carbonate finally obtained?
c) Explain how ammonia is recovered and recycled?
Date posted: May 21, 2019. Answers (1)
- The peaks below show the mass spectrum of element X.
Calculate the relative atomic mass of X.(Solved)
The peaks below show the mass spectrum of element X.

Calculate the relative atomic mass of X.
Date posted: May 21, 2019. Answers (1)
- State two uses of Argon.(Solved)
State two uses of Argon.
Date posted: May 21, 2019. Answers (1)
- Solutions can be classified as acids bases or neutral. The table below shows solutions and their pH values.(Solved)
Solutions can be classified as acids bases or neutral. The table below shows solutions and their pH values.

(i) Select any pair that would react to form a solution of pH 7
(ii) Identify two solutions that would react with Aluminium hydroxide. Explain.
Date posted: May 21, 2019. Answers (1)
- Study the following equilibrium reaction. Suggest two ways of increasing the yield of A2B.(Solved)
Study the following equilibrium reaction
 .
.
Suggest two ways of increasing the yield of A2B.
Date posted: May 21, 2019. Answers (1)
- D grams of potassium hydroxide were dissolved in distilled water to make 100cm3 of
solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for...(Solved)
D grams of potassium hydroxide were dissolved in distilled water to make 100cm3 of
solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for complete neutralization.Calculate the mass D of potassium hydroxide.

(relative formula of KOH=56)
Date posted: May 21, 2019. Answers (1)
- Element A has atomic mass 23 and element B atomic mass 7 and also have 12 neutrons and 4 neutrons respectively.(Solved)
Element A has atomic mass 23 and element B atomic mass 7 and also have 12 neutrons and 4 neutrons respectively.
a) Write the electron arrangement of A and B
b) Which element has higher ionization energy? Explain
Date posted: May 21, 2019. Answers (1)