- Blue litmus turns red while the red litmus unchanged.
- Hydrolysis occurred
sharon kalunda answered the question on May 21, 2019 at 11:41
- Is dissolving of aluminium chloride in water a physical or chemical process? Explain(Solved)
Is dissolving of aluminium chloride in water a physical or chemical process? Explain
Date posted: May 21, 2019. Answers (1)
- During electrolysis of copper (ii) sulphate solution using graphite electrodes, a current of 2
amperes was passed for 15 minutes. Determine the mass of the products...(Solved)
During electrolysis of copper (ii) sulphate solution using graphite electrodes, a current of 2
amperes was passed for 15 minutes. Determine the mass of the products at the cathode.
(1F=96,500C Cu=63.5)
Date posted: May 21, 2019. Answers (1)
- Explain why graphite conducts electricity while diamond does not.(Solved)
Explain why graphite conducts electricity while diamond does not.
Date posted: May 21, 2019. Answers (1)
- The following pairs of compounds were reacted together and the maximum temperature rise
recorded for each reaction.
A- 50 cm3 of 2M ammonia solution and 50 cm3...(Solved)
The following pairs of compounds were reacted together and the maximum temperature rise
recorded for each reaction.
A- 50 cm3 of 2M ammonia solution and 50 cm3 of 2M ethanoic acid.
B- 50 cm3 of 2M sodium hydroxide and 50 cm3 of 2M hydrochloric acid.
C- 50 cm3 of 2M sodium hydroxide and 50 cm3 of 2M ethanoic acid.
(a) State the pair which showed:-
(i) the highest temperature rise.
(ii) the lowest temperature rise.
(b) Explain your answers above.
Date posted: May 21, 2019. Answers (1)
- Radium 226, whose atomic number is 88, undergoes beta decay to form a new element X.Write an equation for this change.(Solved)
Radium 226, whose atomic number is 88, undergoes beta decay to form a new element X.Write an equation for this change.
Date posted: May 21, 2019. Answers (1)
- The table below shows the number of valence electrons in elements D, E and F.
(i) Explain why D and E would not be expected to...(Solved)
The table below shows the number of valence electrons in elements D, E and F.
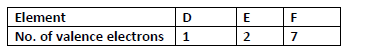
(i) Explain why D and E would not be expected to react together to form a compound.
(ii) Write a chemical equation to show the effect of heat on a carbonate of E.
Date posted: May 21, 2019. Answers (1)
- The structure below shows the repeat unit showed in a polymer.
(i) Name the polymer.
(ii) Draw the structures of the two monomers forming the polymer.(Solved)
The structure below shows the repeat unit showed in a polymer.
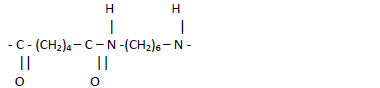
(i) Name the polymer.
(ii) Draw the structures of the two monomers forming the polymer.
Date posted: May 21, 2019. Answers (1)
- Name the solution and the catalyst used in preparation of oxygen in the laboratory.(Solved)
Name the solution and the catalyst used in preparation of oxygen in the laboratory.
Date posted: May 21, 2019. Answers (1)
- When 31.2g of hydrated. Aluminium oxide ( Al2O3XH2O) was heated to a constant mass of 20.6g of Aluminium oxide ( Al2O3) was obtained. Determine the value...(Solved)
When 31.2g of hydrated. Aluminium oxide ( Al2O3XH2O) was heated to a constant
mass of 20.6g of Aluminium oxide ( Al2O3) was obtained. Determine the value of x in
hydrated oxide.(Al= 27.0, O=16.0, H=1.0)
Date posted: May 21, 2019. Answers (1)
- Aluminium is not used for marine purpose.Give a reason.(Solved)
Aluminium is not used for marine purpose.Give a reason.
Date posted: May 21, 2019. Answers (1)
- Carbon is not used for the reduction of Aluminium oxides.Give a reason(Solved)
Carbon is not used for the reduction of Aluminium oxides.Give a reason
Date posted: May 21, 2019. Answers (1)
- The flow chart below shows industrial extraction Aluminium metal. Study it and answer the questions that follow.
(i) Explain how process T is carried out.
(ii)...(Solved)
The flow chart below shows industrial extraction Aluminium metal. Study it and answer the questions that follow.

(i) Explain how process T is carried out.
(ii) Name residue P, give a reason.
(iii) Explain why it is necessary to heat Aluminium oxide in presence of cryolite before
electrolysis is carried out.
Date posted: May 21, 2019. Answers (1)
- Describe how sulphuric acid is manufactured from sulphur (VI) oxide.(Solved)
Describe how sulphuric acid is manufactured from sulphur (VI) oxide.
Date posted: May 21, 2019. Answers (1)
- By using a chemical test, how can you distinguish H2S(g) and SO2(g).(Solved)
By using a chemical test, how can you distinguish H2S(g) and SO2(g).
Date posted: May 21, 2019. Answers (1)
- Study the diagram below and answer the questions that follow
(i) Identify the reagents
(ii) Name the yellow solid.(Solved)
Study the diagram below and answer the questions that follow
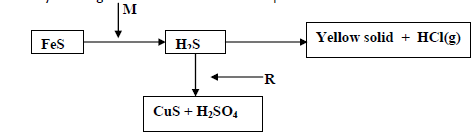
a)(i) Identify the reagents M and R
(ii) Name the yellow solid.
b) What would be the effect of the yield of sulphur (VI) oxide when
(i) Increasing the concentration of oxygen.
(ii) Increasing the temperature .
Date posted: May 21, 2019. Answers (1)
- The diagram below shows the preparation of nitric acid.
a) Name solid A.
b) Under what conditions does sulphuric acid react with solid A.
c) What is the...(Solved)
The diagram below shows the preparation of nitric acid.
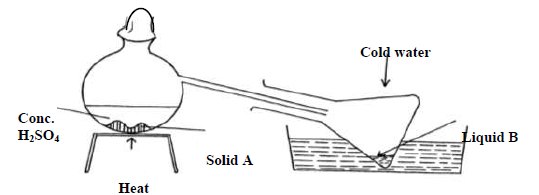
a) Name solid A.
b) Under what conditions does sulphuric acid react with solid A.
c) What is the colour of liquid B
d) What is the purpose of cold water
e) 1 cm3 of liquid B was diluted with distilled water and a few drops of copper turnings dropped into it A colourless gas and later brown gas were produced.
(i) Name the colourless gas
(ii) Name the brown gas formed?
(iii) Give an equation for the formation of the brown gas
Date posted: May 21, 2019. Answers (1)
- Elements V,W and X have atomic number 17,19 and 20 respectively.
(a) What is the valencies of V and W respectively.
(b) To which groups of the...(Solved)
Elements V,W and X have atomic number 17,19 and 20 respectively.
(a) What is the valencies of V and W respectively.
(b) To which groups of the periodic table do V, and X belong.
(c) In which periods do elements V and W lie.?
(d) Which of the three elements is a non-metal?
(e) Write down the formula of the compounds formed when:
(i) V reacts with W
(ii) X reacts with Oxygen
f) How many
(i) Neutrons does V have? if its mass number is 35
(ii) Protons does W have ?
Date posted: May 21, 2019. Answers (1)
- In the preparation of magnesium carbonate magnesium was burnt in air and the product collected.Dilute sulphuric acid was added and the mixture filtered and cooled....(Solved)
In the preparation of magnesium carbonate magnesium was burnt in air and the product collected.Dilute sulphuric acid was added and the mixture filtered and cooled. Sodium carbonate was added to the filtrate and the content filtered. The residue was washed and dried to give a white powder.
a) Give the chemical name of the product formed when magnesium burns in air
b) Write a chemical equation for the formation of product.
c) (i) Name filtrate collected after sodium carbonate was added
(ii) Name the white powder.
d) Write chemical equation for the reaction between product in (a) and acid.
e) Name the ions present in the filtrate after addition of sodium carbonate.
f) Write an ionic equation to show the formation of the white powder
g) Write an equation to show what happened when white powder is strongly heated.
Date posted: May 21, 2019. Answers (1)
- The scheme below shows some products that can be obtained starting from ethene.
(i) Name the compounds
(ii) Name the process
(iii) State one condition necessary...(Solved)
The scheme below shows some products that can be obtained starting from ethene.
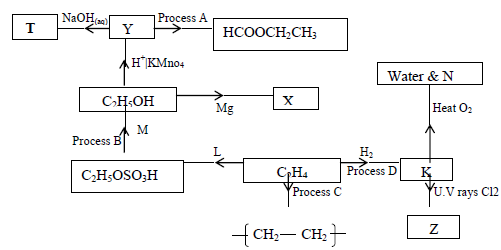
(i) Name the compounds M,N,T,X,Y and Z
(ii) Name the process B,C and D
(iii) State one condition necessary for the processes in (ii) above to take place.
Date posted: May 21, 2019. Answers (1)
- Filter paper dipped in acidified Potassium Manganate (VII) were placed in two
separate gas jars A and B containing pentane and Pent-l-ene respectively. Explain
what was observed...(Solved)
Filter paper dipped in acidified Potassium Manganate (VII) were placed in two
separate gas jars A and B containing pentane and Pent-l-ene respectively. Explain
what was observed in each case.
Date posted: May 21, 2019. Answers (1)