(a) Because Sulphur is non-polar and water does not dissolve non-polar substances
(b) Add water to the mixture and stir then filter and evaporate the filtrate
sharon kalunda answered the question on May 21, 2019 at 13:05
- Explain why potassium is kept under paraffin while phosphorous is kept under water(Solved)
Explain why potassium is kept under paraffin while phosphorous is kept under water
Date posted: May 21, 2019. Answers (1)
- Use the scheme below to answer the questions that follow
a) Identify the solids
i) H -
ii) J -
b) State one laboratory use of Ca(OH)2(aq)(Solved)
Use the scheme below to answer the questions that follow
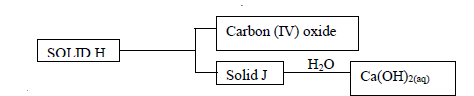
a) Identify the solids
i) H -
ii) J -
b) State one laboratory use of Ca(OH)2(aq)
Date posted: May 21, 2019. Answers (1)
- When an electric current was passed through molten substances M and N in different containers the observations in the table below were made.
Suggest the type...(Solved)
When an electric current was passed through molten substances M and N in different containers the observations in the table below were made.

Suggest the type of bonding present in;
a) Substance M
b) Substance N
Date posted: May 21, 2019. Answers (1)
- 800g of a radioactive isotope decays to 50g in 100 days. Determine the half-life of
this isotope.(Solved)
800g of a radioactive isotope decays to 50g in 100 days. Determine the half-life of
this isotope.
Date posted: May 21, 2019. Answers (1)
- Study the radioactive decay series below and answer the questions below.
(i) Identify the particles emitted in steps III and V
(ii) Write the nuclear...(Solved)
Study the radioactive decay series below and answer the questions below.
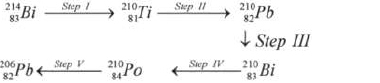
(i) Identify the particles emitted in steps III and V
(ii) Write the nuclear equation for the reaction which takes place in Step I.
Date posted: May 21, 2019. Answers (1)
- Use the information in the table below to answer the questions that follow.
i)Write the cell representation of the cell made of aluminum and iron half-cells.
ii)Calculate...(Solved)
Use the information in the table below to answer the questions that follow.
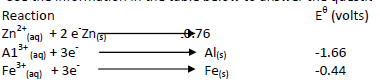
i)Write the cell representation of the cell made of aluminium and iron half-cells.
ii)Calculate the EMF of the cell.
Date posted: May 21, 2019. Answers (1)
- The table below shows the ammeter reading obtained when two different electrolytes of the same concentration were tested.(Solved)
The table below shows the ammeter reading obtained when two different electrolytes of the same concentration were tested.
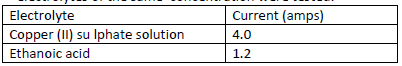
Why does ethanoic acid give a lower reading?
Date posted: May 21, 2019. Answers (1)
- Use the information in the scheme below to answer the questions that follow.
a) Name substance P
b) Give the structure and name of compound Q.
c)...(Solved)
Use the information in the scheme below to answer the questions that follow.
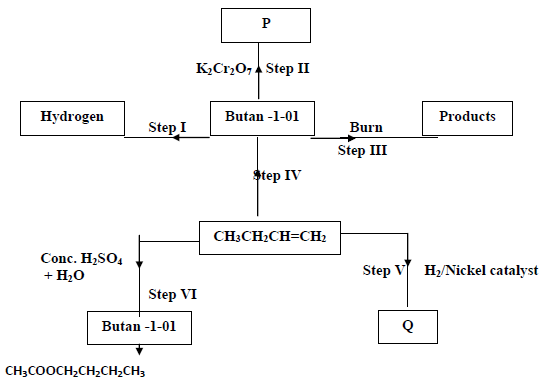
a) Name substance P
b) Give the structure and name of compound Q.
c) Write the equation for the chemical reaction in steps III
d) Name the reagents and conditions necessary for the reaction in
(i) Step IV
Reagents
Conditions
(ii) Step VII
Reagents
Conditions
e) What name is given to the reaction in step VII?
Date posted: May 21, 2019. Answers (1)
- Study the information in the table below and answer the questions that follow (The letters do not represent the actual symbols of the elements).
(i) What...(Solved)
Study the information in the table below and answer the questions that follow (The letters do not represent the actual symbols of the elements).
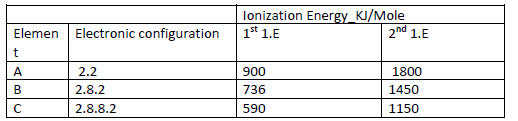
(i) What chemical family do the elements A, B and C belong?
(ii) What is meant by the term ionization energy?
(iii) The 2nd ionization energy is higher that the 1st ionization energy of each. Explain
(Iv) When a piece of element C is placed in cold water, it sinks to the bottom and an
effervescence of a colourless gas that bums explosively is produced. Use a simple diagram to illustrate how this gas can be collected during this experiment.
Date posted: May 21, 2019. Answers (1)
- Study the information given below and answer the questions that follow.
(i) Which elements are non-metals? Give a reason.
(ii) Explain why the melting point of...(Solved)
Study the information given below and answer the questions that follow.
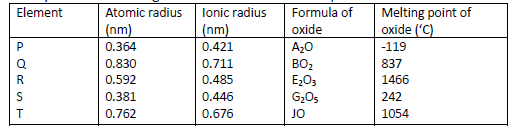
(i) Which elements are non-metals? Give a reason.
(ii) Explain why the melting point of the oxide of R is higher than that of the oxide of S.
(iii) Give two elements that would react vigorously with each other. Explain your answer.
Date posted: May 21, 2019. Answers (1)
- What is the difference between thermosoftening and thermosetting plastics?(Solved)
What is the difference between thermosoftening and thermosetting plastics?
Date posted: May 21, 2019. Answers (1)
- Draw a dot (.) and cross (x) diagram to show bonding in:-
(i) Ammonium ion (NH4)
(ii) Silane (SiH4)
(N=14...(Solved)
Draw a dot (.) and cross (x) diagram to show bonding in:-
(i) Ammonium ion (NH4)
(ii) Silane (SiH4)
(N=14 H=1 Si=14)
Date posted: May 21, 2019. Answers (1)
- Lead (ii)nitrate was heated strongly for some time.
(i) State two observations made during heating.
(ii) Write an equation for the reaction.(Solved)
Lead (ii)nitrate was heated strongly for some time.
(i) State two observations made during heating.
(ii) Write an equation for the reaction.
Date posted: May 21, 2019. Answers (1)
- Consider the following electrochemical cell.
(i) Name the electrodes for the above cell.
(ii) Write the electrodes for the above cell
(iii) Name a possible salt...(Solved)
Consider the following electrochemical cell.

(i) Name the electrodes for the above cell.
(ii) Write the electrodes for the above cell
(iii) Name a possible salt bridge.
Date posted: May 21, 2019. Answers (1)
- A volume of nitrogen gas diffuses through a porous pot in 70 seconds. How long would it take
400cm3 of carbon (iv) oxide to diffuse through...(Solved)
A volume of nitrogen gas diffuses through a porous pot in 70 seconds. How long would it take
400cm3 of carbon (iv) oxide to diffuse through the same porous pot? (C=12 O=16 N=14)
Date posted: May 21, 2019. Answers (1)
- When a few drops of aqueous ammonia were added to copper (ii) chloride solution, a light blue
precipitate was formed. On addition of excess ammonia solution,...(Solved)
When a few drops of aqueous ammonia were added to copper (ii) chloride solution, a light blue
precipitate was formed. On addition of excess ammonia solution, a deep blue solution was formed.
(a) Identify the substance responsible for the:-
(i) light blue precipitate.
(ii) deep blue solution.
(b) Write an equation for the reaction leading to observation in (a) (ii) above.
Date posted: May 21, 2019. Answers (1)
- Pieces of blue and red litmus papers were placed into a beaker containing water into which
Aluminium Chloride had been dissolved.State the observations made on the...(Solved)
Pieces of blue and red litmus papers were placed into a beaker containing water into which
Aluminium Chloride had been dissolved.State the observations made on the papers. Explain your answer.
Date posted: May 21, 2019. Answers (1)
- Is dissolving of aluminium chloride in water a physical or chemical process? Explain(Solved)
Is dissolving of aluminium chloride in water a physical or chemical process? Explain
Date posted: May 21, 2019. Answers (1)
- During electrolysis of copper (ii) sulphate solution using graphite electrodes, a current of 2
amperes was passed for 15 minutes. Determine the mass of the products...(Solved)
During electrolysis of copper (ii) sulphate solution using graphite electrodes, a current of 2
amperes was passed for 15 minutes. Determine the mass of the products at the cathode.
(1F=96,500C Cu=63.5)
Date posted: May 21, 2019. Answers (1)
- Explain why graphite conducts electricity while diamond does not.(Solved)
Explain why graphite conducts electricity while diamond does not.
Date posted: May 21, 2019. Answers (1)