-
75g of a saturated solution contains 30g of salt calculate
a) The solubility of the salt
b) The percentage of the salt in the saturated solution
(Solved)
75g of a saturated solution contains 30g of salt calculate
a) The solubility of the salt
b) The percentage of the salt in the saturated solution
Date posted:
May 21, 2019
.
Answers (1)
-
Draw a dot (.) and cross (x) diagram to show bonding in:-
(i) Ammonium ion (NH4)
(ii) Silane (SiH4)
(N=14...
(Solved)
Draw a dot (.) and cross (x) diagram to show bonding in:-
(i) Ammonium ion (NH4)
(ii) Silane (SiH4)
(N=14 H=1 Si=14)
Date posted:
May 21, 2019
.
Answers (1)
-
Explain why graphite conducts electricity while diamond does not.
(Solved)
Explain why graphite conducts electricity while diamond does not.
Date posted:
May 21, 2019
.
Answers (1)
-
When 31.2g of hydrated. Aluminium oxide ( Al2O3XH2O) was heated to a constant mass of 20.6g of Aluminium oxide ( Al2O3) was obtained. Determine the value...
(Solved)
When 31.2g of hydrated. Aluminium oxide ( Al2O3XH2O) was heated to a constant
mass of 20.6g of Aluminium oxide ( Al2O3) was obtained. Determine the value of x in
hydrated oxide.(Al= 27.0, O=16.0, H=1.0)
Date posted:
May 21, 2019
.
Answers (1)
-
The flow chart below shows industrial extraction Aluminium metal. Study it and answer the questions that follow.
(i) Explain how process T is carried out.
(ii)...
(Solved)
The flow chart below shows industrial extraction Aluminium metal. Study it and answer the questions that follow.

(i) Explain how process T is carried out.
(ii) Name residue P, give a reason.
(iii) Explain why it is necessary to heat Aluminium oxide in presence of cryolite before
electrolysis is carried out.
Date posted:
May 21, 2019
.
Answers (1)
-
Describe how sulphuric acid is manufactured from sulphur (VI) oxide.
(Solved)
Describe how sulphuric acid is manufactured from sulphur (VI) oxide.
Date posted:
May 21, 2019
.
Answers (1)
-
In the preparation of magnesium carbonate magnesium was burnt in air and the product collected.Dilute sulphuric acid was added and the mixture filtered and cooled....
(Solved)
In the preparation of magnesium carbonate magnesium was burnt in air and the product collected.Dilute sulphuric acid was added and the mixture filtered and cooled. Sodium carbonate was added to the filtrate and the content filtered. The residue was washed and dried to give a white powder.
a) Give the chemical name of the product formed when magnesium burns in air
b) Write a chemical equation for the formation of product.
c) (i) Name filtrate collected after sodium carbonate was added
(ii) Name the white powder.
d) Write chemical equation for the reaction between product in (a) and acid.
e) Name the ions present in the filtrate after addition of sodium carbonate.
f) Write an ionic equation to show the formation of the white powder
g) Write an equation to show what happened when white powder is strongly heated.
Date posted:
May 21, 2019
.
Answers (1)
-
Filter paper dipped in acidified Potassium Manganate (VII) were placed in two
separate gas jars A and B containing pentane and Pent-l-ene respectively. Explain
what was observed...
(Solved)
Filter paper dipped in acidified Potassium Manganate (VII) were placed in two
separate gas jars A and B containing pentane and Pent-l-ene respectively. Explain
what was observed in each case.
Date posted:
May 21, 2019
.
Answers (1)
-
An aqueous solution of Copper (II) Sulphate was electrolysed using platinum electrodes. When a current was passed a gas that relights a glowing splint was...
(Solved)
An aqueous solution of Copper (II) Sulphate was electrolysed using platinum electrodes. When a current was passed a gas that relights a glowing splint was produced.
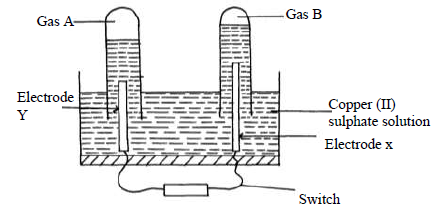
(i) Name the electrode which acts as cathode. Give a reason.
(ii) Write an equation for the reaction at the anode.
d) 0.11g of metal R deposited by electrolysis when a current of 0.03 amperes flow for
99 minutes. ( R =92.) ,(1 Faraday = 96500 C)
(i) Find the number of moles of metal deposited.
(ii) Find the number of moles of electrons passed.
(iii) Determine the value of n in the metallic ion Rn+.
Date posted:
May 21, 2019
.
Answers (1)
-
Study the information below and answer the following questions. A mixture contains three
solid A,B and C. the solubility of these solids in different liquids is...
(Solved)
Study the information below and answer the following questions. A mixture contains three
solid A,B and C. the solubility of these solids in different liquids is as shown below
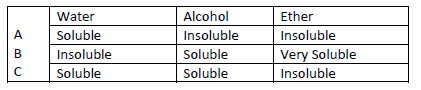
Explain how you will obtain sample C from the mixture.
Date posted:
May 21, 2019
.
Answers (1)
-
The peaks below show the mass spectrum of element X.
Calculate the relative atomic mass of X.
(Solved)
The peaks below show the mass spectrum of element X.

Calculate the relative atomic mass of X.
Date posted:
May 21, 2019
.
Answers (1)
-
Solutions can be classified as acids bases or neutral. The table below shows solutions and their pH values.
(Solved)
Solutions can be classified as acids bases or neutral. The table below shows solutions and their pH values.

(i) Select any pair that would react to form a solution of pH 7
(ii) Identify two solutions that would react with Aluminium hydroxide. Explain.
Date posted:
May 21, 2019
.
Answers (1)
-
D grams of potassium hydroxide were dissolved in distilled water to make 100cm3 of
solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for...
(Solved)
D grams of potassium hydroxide were dissolved in distilled water to make 100cm3 of
solution. 50cm3 of the solution required 50cm3 of 2.0M nitric acid for complete neutralization.Calculate the mass D of potassium hydroxide.

(relative formula of KOH=56)
Date posted:
May 21, 2019
.
Answers (1)
-
Element A has atomic mass 23 and element B atomic mass 7 and also have 12 neutrons and 4 neutrons respectively.
(Solved)
Element A has atomic mass 23 and element B atomic mass 7 and also have 12 neutrons and 4 neutrons respectively.
a) Write the electron arrangement of A and B
b) Which element has higher ionization energy? Explain
Date posted:
May 21, 2019
.
Answers (1)
-
When hydrogen chloride gas is dissolved in water the solution formed turns blue litmus paper red but there is no effect on blue litmus paper...
(Solved)
When hydrogen chloride gas is dissolved in water the solution formed turns blue litmus paper red but there is no effect on blue litmus paper when the gas is dissolved in carbon tetra chloride.Explain
Date posted:
May 21, 2019
.
Answers (1)
-
When lead (II) carbonate reacts with dilute hydrochloric acid, very little carbon (iv) oxide is produced.Explain
(Solved)
When lead (II) carbonate reacts with dilute hydrochloric acid, very little carbon (iv) oxide is produced.Explain
Date posted:
May 21, 2019
.
Answers (1)
-
Given the equation for reaction below Calculate
(i) Volume of chlorine at (r.t.p) required to react with 3g of Aluminium (Molar gas volume at r.t.p =...
(Solved)
Given the equation for reaction below

Calculate
(i) Volume of chlorine at (r.t.p) required to react with 3g of Aluminium (Molar gas volume at r.t.p = 24litres, Al = 27, Cl = 35.5).
(ii) Mass of Aluminium chloride formed.
Date posted:
May 21, 2019
.
Answers (1)
-
Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions.
(Solved)
Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions.
Date posted:
May 21, 2019
.
Answers (1)
-
The apparatus below was a set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow.
(i) Write an...
(Solved)
The apparatus below was a set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow.

(i) Write an equation for the reaction that takes place in the gas jar.
(ii) Why is it necessary to have a hot nichrome wire in the gas jar.
Date posted:
May 21, 2019
.
Answers (1)
-
State the particles responsible for conductivity of an electric current in
(i) Solution
(ii) A metal
(Solved)
State the particles responsible for conductivity of an electric current in
(i) Solution
(ii) A metal
Date posted:
May 21, 2019
.
Answers (1)