- If aqueous lead (II) nitrate is added to aqueous solution potassium iodide, a bright yellow
precipitate is formed.
(i) Write down the formula of the precipitate formed.
(ii)...(Solved)
If aqueous lead (II) nitrate is added to aqueous solution potassium iodide, a bright yellow
precipitate is formed.
(i) Write down the formula of the precipitate formed.
(ii) Write an ionic equation for the reaction above.
Date posted: May 22, 2019. Answers (1)
- Study the information in the table below and answer the questions that follow. The letters
do not represent the actual symbols of the elements.(Solved)
Study the information in the table below and answer the questions that follow. The letters
do not represent the actual symbols of the elements.

(i) Write the formulae of carbonate R and M
(ii) Describe how the carbonate of M can be obtained from a mixture of carbonate R and
M.
(iii) R is more reactive than L. Explain
Date posted: May 22, 2019. Answers (1)
- The diagram below shows a set-up of apparatus used to separate miscible liquids.
(a) Name the parts labelled A and B
(b) State the function of...(Solved)
The diagram below shows a set-up of apparatus used to separate miscible liquids.
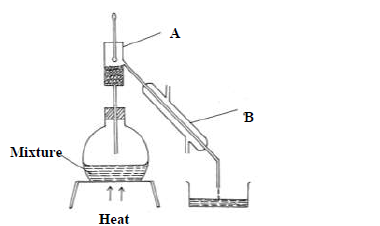
(a) Name the parts labelled A and B
(b) State the function of the part labeled A.
(c) State the property of the mixture that makes it suitable to be separated by the
method above.
Date posted: May 22, 2019. Answers (1)
- The table represents results on four samples of water. Study it an answer the
question that follows.
Which sample is likely to be temporary hard water? Explain(Solved)
The table represents results on four samples of water. Study it an answer the
question that follows.
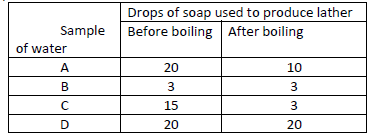
Which sample is likely to be temporary hard water? Explain
Date posted: May 22, 2019. Answers (1)
- Use the flow chart below to answer the questions that follow
a) Name the following;
i) Gas L
ii) Gas H
iii) K
b) Name the processes...(Solved)
Use the flow chart below to answer the questions that follow
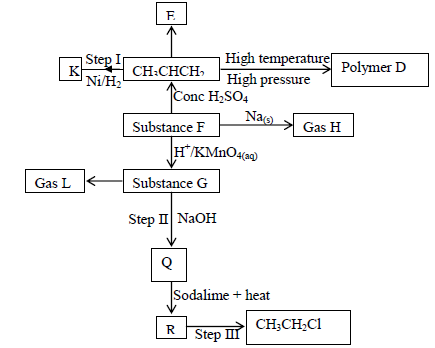
a) Name the following;
i) Gas L
ii) Gas H
iii) K
b) Name the processes involved in the following steps
i) Step I
ii) Step II
iii) Step III
c) Write a chemical equation for the complete combustion of substance F
d) Name the condition and reagents in step III
i) Condition
ii) Reagent
e) Calculate the mass of salt Q that would be formed by using 21.9kg of G when
it reacts with excess sodium hydroxide
(C = 120, H = 1.0, Na = 23.0, O = 16.0)
Date posted: May 22, 2019. Answers (1)
- Below is a list of potential differences obtained when metal P, Q, R, S and T are used
in the following electrochemical cell(Solved)
Below is a list of potential differences obtained when metal P, Q, R, S and T are used
in the following electrochemical cell
Metal (s) / Metal ions // Copper (II) ions / copper (s)
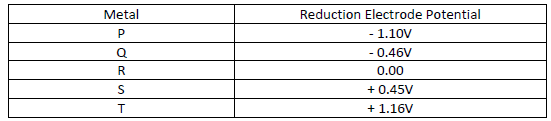
i) Which metal is likely to be Copper. Explain.
ii) Identify the strongest reducing agent.
iii) Which two half – cells would be combined to produce the highest voltage?.
iv) Give a cell representation of the cell in F (iii) above.
Date posted: May 22, 2019. Answers (1)
- Give any two uses of ethene gas(Solved)
Give any two uses of ethene gas
Date posted: May 22, 2019. Answers (1)
- The grid below represents part of the periodic table. Study it and answer the
questions that follow. (The letters are not the actual symbols of the...(Solved)
The grid below represents part of the periodic table. Study it and answer the
questions that follow. (The letters are not the actual symbols of the elements)

i) Select the element in period three which has the shortest atomic radius. Give a reason
for your answer.
ii) Using dots (.) and crosses (x) to represent outermost electrons, draw a diagram to
show the bonding in the compound formed when chlorine reacts with element X
iii) When three liters of chlorine gas were completely reacted with element Y, 11.85g of
the product were formed. Calculate the relative atomic mass of element Y
(R.A.M of chlorine = 35.5, molar gas volume = 24 liters)
Date posted: May 21, 2019. Answers (1)
- Use the information in the table below to answer the questions that follow
i) Are the members in this group likely to be conductor or non...(Solved)
Use the information in the table below to answer the questions that follow
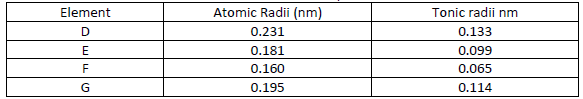
i) Are the members in this group likely to be conductor or non – conductors?
ii) Which element would have the lowest atomic number? Explain.
Date posted: May 21, 2019. Answers (1)
- A volume of 80cm3 of a mixture of propane (C3H8) and oxygen were ignited in an experiment.The products were cooled and passed through an aqueous...(Solved)
A volume of 80cm3 of a mixture of propane (C3H8) and oxygen were ignited in an experiment.The products were cooled and passed through an aqueous sodium hydroxide. The final volume
was reduced by 30cm3
a) Write the equation for the combustion of propane
b) Determine the volume of;
i) The component of the original mixture
ii) Residual oxygen
Date posted: May 21, 2019. Answers (1)
- Differentiate between empirical and molecular formula(Solved)
Differentiate between empirical and molecular formula
Date posted: May 21, 2019. Answers (1)
- Study the figure below and answer the questions that follow
Identify the radiations A, B and C(Solved)
Study the figure below and answer the questions that follow
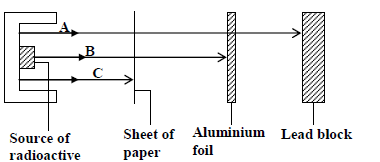
Identify the radiations A, B and C
Date posted: May 21, 2019. Answers (1)
- Draw the structural formula of ethanoic acid(Solved)
Draw the structural formula of ethanoic acid
Date posted: May 21, 2019. Answers (1)
- Give a reasons why ethanoic acid has a higher boiling point than ethanol which has the same
number of Carbon atoms(Solved)
Give a reasons why ethanoic acid has a higher boiling point than ethanol which has the same
number of Carbon atoms
Date posted: May 21, 2019. Answers (1)
- Define oxidation and reduction in terms of electrons.(Solved)
Define oxidation and reduction in terms of electrons.
Date posted: May 21, 2019. Answers (1)
- 75g of a saturated solution contains 30g of salt calculate
a) The solubility of the salt
b) The percentage of the salt in the saturated solution(Solved)
75g of a saturated solution contains 30g of salt calculate
a) The solubility of the salt
b) The percentage of the salt in the saturated solution
Date posted: May 21, 2019. Answers (1)
- Three brands of inks M, N and O were suspected to be contaminated with substance P.
The result is shown below;
i) Which ink was contaminated with...(Solved)
Three brands of inks M, N and O were suspected to be contaminated with substance P.
The result is shown below;
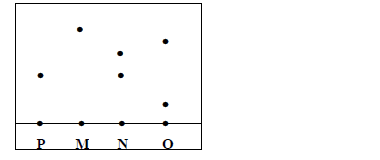
i) Which ink was contaminated with substance P
ii) Name the ink which was pure
iii) Identify the other ink which was not pure
Date posted: May 21, 2019. Answers (1)
- Name three sub – atomic particles found in an atom and state where they are found(Solved)
Name three sub – atomic particles found in an atom and state where they are found
Date posted: May 21, 2019. Answers (1)
- A fixed mass of an ideal gas occupies 200cm3 at a pressure of 740 mmHg.Calculate the volume of the gas at 77-mmHg pressure.(Solved)
A fixed mass of an ideal gas occupies 200cm3 at a pressure of 740 mmHg.Calculate the volume of the gas at 77-mmHg pressure.
Date posted: May 21, 2019. Answers (1)
- Ammionia gas is prepared by harber process according to the equation below
Complete the table below by stating the effect of equilibrium when the following conditions...(Solved)
Ammionia gas is prepared by harber process according to the equation below
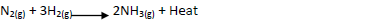
Complete the table below by stating the effect of equilibrium when the following conditions are
applied. Give explanation in each case

Date posted: May 21, 2019. Answers (1)