- The following is a list of pH values of some substance:(i) Strong acid (ii)Weak base(Solved)
The following is a list of pH values of some substance:

Identify:
(i) Strong acid
(ii)Weak base
Date posted: May 22, 2019. Answers (1)
- Distinguishing between weak and strong alkali(Solved)
Distinguishing between weak and strong alkali
Date posted: May 22, 2019. Answers (1)
- The structure below represents a polymer
(a) State the name of the polymer
(b) State one industrial use of the polymer(Solved)
The structure below represents a polymer
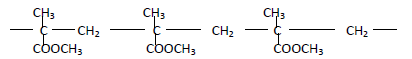
(a) State the name of the polymer
(b) State one industrial use of the polymer
Date posted: May 22, 2019. Answers (1)
- A dry gas X was passed over heated lead (II) oxide. A grey residue and a gas Y were formed.
The gas Y has no effect...(Solved)
A dry gas X was passed over heated lead (II) oxide. A grey residue and a gas Y were formed.
The gas Y has no effect on red litmus paper and does not support combustion. Identity:
(i) Gas X
(ii) Gas Y
Date posted: May 22, 2019. Answers (1)
- 20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.(Solved)
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
Date posted: May 22, 2019. Answers (1)
- Give one industrial application of solvent extraction(Solved)
Give one industrial application of solvent extraction
Date posted: May 22, 2019. Answers (1)
- The table below shows information about three substances K, L and M. Study it and answer the
questions that follow:
Describe how you will separate the three...(Solved)
The table below shows information about three substances K, L and M. Study it and answer the
questions that follow:
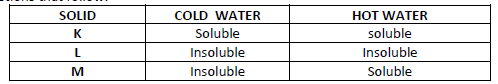
Describe how you will separate the three solids from a mixture of three.
Date posted: May 22, 2019. Answers (1)
- The table below gives the standard electrode potentials for a number of half reactions
(i) Write a cell representation of the two half cells which would...(Solved)
The table below gives the standard electrode potentials for a number of half reactions
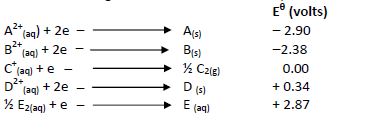
(i) Write a cell representation of the two half cells which would produce the highest
e.m.f
(ii) Calculate the e.m.f of the cell above.
Date posted: May 22, 2019. Answers (1)
- In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another containing 70cm3 of hydrogen sulphide gas. The two gases reacted according...(Solved)
In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another
containing 70cm3 of hydrogen sulphide gas. The two gases reacted according to the equation
below to form 80cm3 of hydrogen chloride gas.
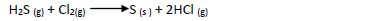
(a) Using oxidation number identify the oxidizing agent
(b) Calculate the percentage yield of hydrogen chloride gas
Date posted: May 22, 2019. Answers (1)
- Apart from using combustion, bromine liquid or potassium manganate (VII) solution,describe how you would distinguish between ethene and ethyne by chemical means.(Solved)
Apart from using combustion, bromine liquid or potassium manganate (VII) solution, describe how you would distinguish between ethene and ethyne by chemical means.
Date posted: May 22, 2019. Answers (1)
- Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution...(Solved)
Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution of concentrated sodium chloride for 30 minutes.
(Na = 23, Cl = 35.5, Faraday constant = 96500, M.G V = 22.4dm3)
Date posted: May 22, 2019. Answers (1)
- The table below shows the number of drops of soap solution needed to lather with 10cm3 of
water.
(a) Identify the anions likely to be in:A and...(Solved)
The table below shows the number of drops of soap solution needed to lather with 10cm3 of
water.
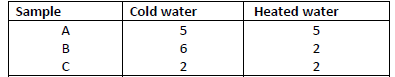
(a) Identify the anions likely to be in:A and B
(b) State two methods used in removing temporary hardness of water.
Date posted: May 22, 2019. Answers (1)
- Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the
equation below:
The reaction is carried out in the presence of a chromium catalyst...(Solved)
Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the
equation below:
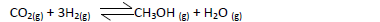
The reaction is carried out in the presence of a chromium catalyst at 700K and 30kPa. Under
these conditions, an equilibrium is reached when 2 % of the carbon (iv) oxide is converted to
methanol?
(a) How does the rate of forward reaction compare with that of the reverse reaction
when 2% of the carbon(iv)oxide is converted to methanol?
(b) Explain how each of the following conditions would affect the yield of methanol:
(i) Reduction in pressure
(ii) Using a more efficient catalyst.
(c) If the reaction is carried out at 500K and 30kPa the percentage of carbon (iv) oxide
converted is higher than 2%. What is the sign of ΔH for the reaction? Explain.
Date posted: May 22, 2019. Answers (1)
- Study the scheme given below and answer questions that follow.
(i) Name the reagent used in
Step I
Step I
Step III
(ii) Write an equation for...(Solved)
Study the scheme given below and answer questions that follow.
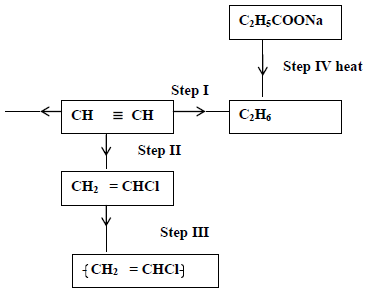
(i) Name the reagent used in
Step I
Step II
Step III
(ii) Write an equation for complete combustion of CH≡CH.
(iii) Explain one disadvantage of the continued use of items in step III.
Date posted: May 22, 2019. Answers (1)
- Study the information in the table below and answer the questions that follow.
(i) Write the general formula of the hydrocarbons in the table.
(ii) Predict...(Solved)
Study the information in the table below and answer the questions that follow.
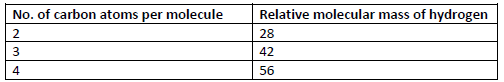
(i) Write the general formula of the hydrocarbons in the table.
(ii) Predict the relative molecular mass of the hydrocarbon with 5 carbon atoms.
(iii) Determine the molecular formula of the hydrocarbon in (ii) and draw its structural
formula.
Date posted: May 22, 2019. Answers (1)
- Describe how the mixture of Ammonium chloride, sodium chloride and lead II chloride can be separated if all the components of the mixture are to...(Solved)
Describe how the mixture of Ammonium chloride, sodium chloride and lead II chloride can be separated if all the components of the mixture are to be recovered.
Date posted: May 22, 2019. Answers (1)
- The diagram below represents an arrangement for a large scale manufacture of ethanol for domestic consumption.
(i) Name the process by which ethanol is obtained from...(Solved)
The diagram below represents an arrangement for a large scale manufacture of ethanol for domestic consumption.
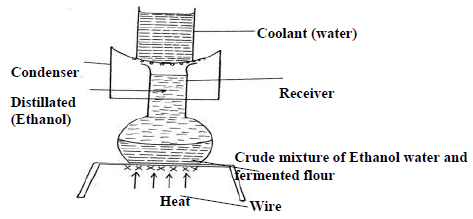
(i) Name the process by which ethanol is obtained from the crude oil.
(ii) Suggest two reasons why water is a coolant in this process.
(iii) Why is it possible to separate ethanol from the mixture by this process.
Date posted: May 22, 2019. Answers (1)
- Study the energy level below and answer the questions that follow.
(i) State and explain whether the reaction represented in the diagram is endothermic or
exothermic.
(ii)...(Solved)
Study the energy level below and answer the questions that follow.
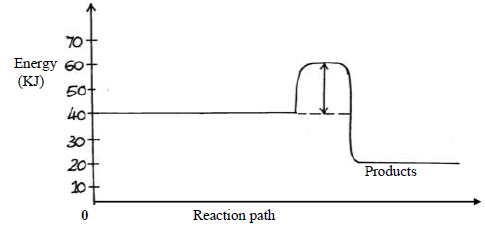
(i) State and explain whether the reaction represented in the diagram is endothermic or
exothermic.
(ii) From the diagram, determine; the activation energy
Date posted: May 22, 2019. Answers (1)
- Consider the reaction represented by the equation:
Explain the effect of the following on the reaction;
(a) An increase in pressure
(b) Increase in temperature(Solved)
Consider the reaction represented by the equation:
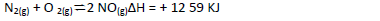
Explain the effect of the following on the reaction;
(a) An increase in pressure
(b) Increase in temperature
Date posted: May 22, 2019. Answers (1)
- In an experiment, a few drops of concentrated nitric (IV)acid were added to aqueous Iron (II)
sulphate in a test tube. excess sodium hydroxide solution was...(Solved)
In an experiment, a few drops of concentrated nitric (IV)acid were added to aqueous Iron (II)
sulphate in a test tube. excess sodium hydroxide solution was then added to the mixture.
(a) State the observations that were made when:
(i) Concentrated nitric (V) acid was added to aqueous Iron (II) sulphate.
(ii) Excess sodium hydroxide was added to the mixture.
(b) Write an ionic equation for the reaction that occurred in a(ii) above.
Date posted: May 22, 2019. Answers (1)