a) Separating funnel
b) Liquid B
c) Decantation
sharon kalunda answered the question on May 22, 2019 at 12:55
- The diagram below shows a Bunsen burner which is used as a source of heat in the
laboratory.(Solved)
The diagram below shows a Bunsen burner which is used as a source of heat in the
laboratory.
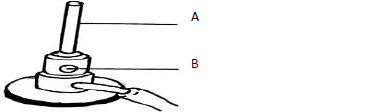
Name the parts labeled: (
A
B
Date posted: May 22, 2019. Answers (1)
- State the type of unemployment described below
i. Experienced where demand for goods and services is seasonal
ii. Occurs when a person seems to be employed...(Solved)
State the type of unemployment described below
i. Experienced where demand for goods and services is seasonal
ii. Occurs when a person seems to be employed but is mainly under
utilized
iii. Occurs when jobs are available but one does not have the required
qualifications
iv. Caused by Economic factors leading to poor performance of the
Economy
Date posted: May 22, 2019. Answers (1)
- Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.(Solved)
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
Date posted: May 22, 2019. Answers (1)
- The flow chart below shows the manufacture of a cleansing agent.
(i) Identify each of the substance D and L
(ii) Give one advantage of using this...(Solved)
The flow chart below shows the manufacture of a cleansing agent.
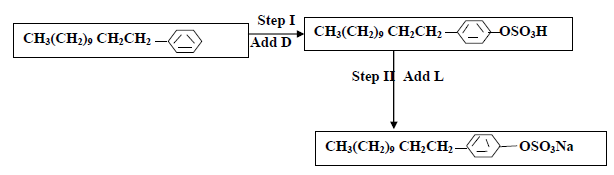
(i) Identify each of the substance D and L
(ii) Give one advantage of using this cleansing agent over ordinary soap
(iii) What is the effect of the above cleansing agent to the environment.
Date posted: May 22, 2019. Answers (1)
- The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that...(Solved)
The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that follow:
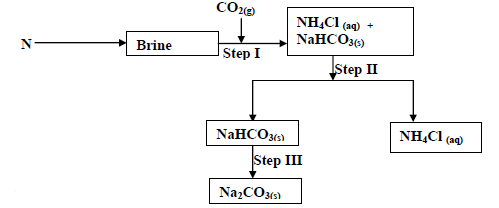
(a) Name substance N
(b) Name the process taking place in
(i) Step II
(ii) Step III
(c) Write an equation for the react producing sodium carbonate.
Date posted: May 22, 2019. Answers (1)
- Draw the structure of a sulphur molecule(Solved)
Draw the structure of a sulphur molecule
Date posted: May 22, 2019. Answers (1)
- The following is a list of pH values of some substance:(i) Strong acid (ii)Weak base(Solved)
The following is a list of pH values of some substance:

Identify:
(i) Strong acid
(ii)Weak base
Date posted: May 22, 2019. Answers (1)
- Distinguishing between weak and strong alkali(Solved)
Distinguishing between weak and strong alkali
Date posted: May 22, 2019. Answers (1)
- The structure below represents a polymer
(a) State the name of the polymer
(b) State one industrial use of the polymer(Solved)
The structure below represents a polymer
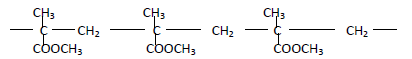
(a) State the name of the polymer
(b) State one industrial use of the polymer
Date posted: May 22, 2019. Answers (1)
- A dry gas X was passed over heated lead (II) oxide. A grey residue and a gas Y were formed.
The gas Y has no effect...(Solved)
A dry gas X was passed over heated lead (II) oxide. A grey residue and a gas Y were formed.
The gas Y has no effect on red litmus paper and does not support combustion. Identity:
(i) Gas X
(ii) Gas Y
Date posted: May 22, 2019. Answers (1)
- 20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.(Solved)
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
Date posted: May 22, 2019. Answers (1)
- Give one industrial application of solvent extraction(Solved)
Give one industrial application of solvent extraction
Date posted: May 22, 2019. Answers (1)
- The table below shows information about three substances K, L and M. Study it and answer the
questions that follow:
Describe how you will separate the three...(Solved)
The table below shows information about three substances K, L and M. Study it and answer the
questions that follow:
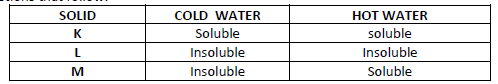
Describe how you will separate the three solids from a mixture of three.
Date posted: May 22, 2019. Answers (1)
- The table below gives the standard electrode potentials for a number of half reactions
(i) Write a cell representation of the two half cells which would...(Solved)
The table below gives the standard electrode potentials for a number of half reactions
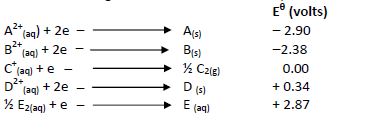
(i) Write a cell representation of the two half cells which would produce the highest
e.m.f
(ii) Calculate the e.m.f of the cell above.
Date posted: May 22, 2019. Answers (1)
- In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another containing 70cm3 of hydrogen sulphide gas. The two gases reacted according...(Solved)
In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another
containing 70cm3 of hydrogen sulphide gas. The two gases reacted according to the equation
below to form 80cm3 of hydrogen chloride gas.
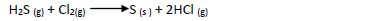
(a) Using oxidation number identify the oxidizing agent
(b) Calculate the percentage yield of hydrogen chloride gas
Date posted: May 22, 2019. Answers (1)
- Apart from using combustion, bromine liquid or potassium manganate (VII) solution,describe how you would distinguish between ethene and ethyne by chemical means.(Solved)
Apart from using combustion, bromine liquid or potassium manganate (VII) solution, describe how you would distinguish between ethene and ethyne by chemical means.
Date posted: May 22, 2019. Answers (1)
- Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution...(Solved)
Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution of concentrated sodium chloride for 30 minutes.
(Na = 23, Cl = 35.5, Faraday constant = 96500, M.G V = 22.4dm3)
Date posted: May 22, 2019. Answers (1)
- The table below shows the number of drops of soap solution needed to lather with 10cm3 of
water.
(a) Identify the anions likely to be in:A and...(Solved)
The table below shows the number of drops of soap solution needed to lather with 10cm3 of
water.
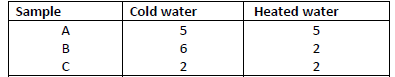
(a) Identify the anions likely to be in:A and B
(b) State two methods used in removing temporary hardness of water.
Date posted: May 22, 2019. Answers (1)
- Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the
equation below:
The reaction is carried out in the presence of a chromium catalyst...(Solved)
Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the
equation below:
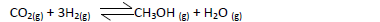
The reaction is carried out in the presence of a chromium catalyst at 700K and 30kPa. Under
these conditions, an equilibrium is reached when 2 % of the carbon (iv) oxide is converted to
methanol?
(a) How does the rate of forward reaction compare with that of the reverse reaction
when 2% of the carbon(iv)oxide is converted to methanol?
(b) Explain how each of the following conditions would affect the yield of methanol:
(i) Reduction in pressure
(ii) Using a more efficient catalyst.
(c) If the reaction is carried out at 500K and 30kPa the percentage of carbon (iv) oxide
converted is higher than 2%. What is the sign of ΔH for the reaction? Explain.
Date posted: May 22, 2019. Answers (1)
- Study the scheme given below and answer questions that follow.
(i) Name the reagent used in
Step I
Step I
Step III
(ii) Write an equation for...(Solved)
Study the scheme given below and answer questions that follow.
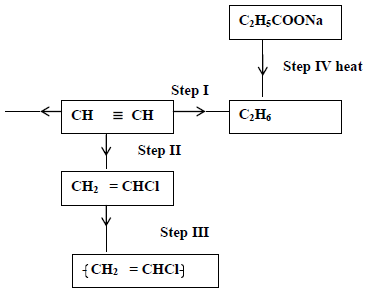
(i) Name the reagent used in
Step I
Step II
Step III
(ii) Write an equation for complete combustion of CH≡CH.
(iii) Explain one disadvantage of the continued use of items in step III.
Date posted: May 22, 2019. Answers (1)