- A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into...(Solved)
A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into a vacuum. An
identical amount of oxygen gas passes through the same aperture in 20 seconds.
Determine the molar mass of gas Z. (O=16, S=32).
Date posted: May 22, 2019. Answers (1)
- Write equations to show the effect of heat on each of the following.
(a) Sodium hydrogen carbonate.
(b) Silver nitrate
(c ) Sodium nitrate(Solved)
Write equations to show the effect of heat on each of the following.
(a) Sodium hydrogen carbonate.
(b) Silver nitrate
(c ) Sodium nitrate
Date posted: May 22, 2019. Answers (1)
- When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the...(Solved)
When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the white
precipitate dissolves to form a colorless solution.
a) Name the white precipitate.
b) Explain using chemical equations why the white precipitate dissolves in excess of
carbon(IV) oxide.
c) What will happen of the above colorless solution is boiled.
Date posted: May 22, 2019. Answers (1)
- When magnesium burns in air, it forms two products. When one of the products
dissolves in water, a colorless gas that turns red litmus paper blue...(Solved)
When magnesium burns in air, it forms two products. When one of the products
dissolves in water, a colorless gas that turns red litmus paper blue is formed.
a) Name the product that dissolves in water to produce a colorless gas.
b) Write an equation for the formation of the colorless gas.
c) State any one use of the colorless gas.
Date posted: May 22, 2019. Answers (1)
- The table below shows elements and their atomic numbers. The letters do not
represent the actual symbols of the elements.
a)From the given letters, select two elements...(Solved)
The table below shows elements and their atomic numbers. The letters do not
represent the actual symbols of the elements.
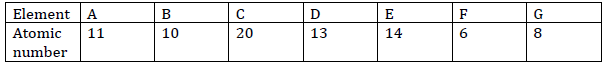
a)From the given letters, select two elements with the same chemical properties.
b) Write the formula of the compound formed when element E reacts with element G.
c) Identify the most stable element and give a reason for your answer.
Date posted: May 22, 2019. Answers (1)
- A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
Identify with reasons two mistakes in the above set-up.(Solved)
A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
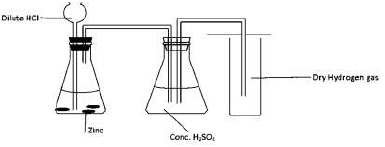
Identify with reasons two mistakes in the above set-up.
Date posted: May 22, 2019. Answers (1)
- The following chromatogram shows the results obtained after separating substances P
and T.(Solved)
The following chromatogram shows the results obtained after separating substances P
and T.
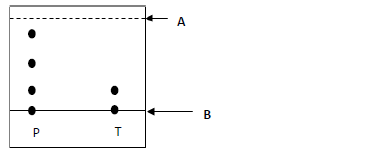
a) Name lines;
A
B
b) Name the possible solvent that can be used in the above process.
c) Which of the two substances is pure?
Date posted: May 22, 2019. Answers (1)
- In an experiment to separate a mixture of two immiscible liquids A and B, a form four
student set the apparatus as shown below.
a) Name the...(Solved)
In an experiment to separate a mixture of two immiscible liquids A and B, a form four
student set the apparatus as shown below.
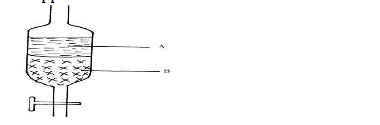
a) Name the above apparatus.
b) Which liquid is denser?
c) Name one other method that can be used to separate the above mixture.
Date posted: May 22, 2019. Answers (1)
- The diagram below shows a Bunsen burner which is used as a source of heat in the
laboratory.(Solved)
The diagram below shows a Bunsen burner which is used as a source of heat in the
laboratory.
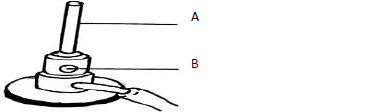
Name the parts labeled: (
A
B
Date posted: May 22, 2019. Answers (1)
- State the type of unemployment described below
i. Experienced where demand for goods and services is seasonal
ii. Occurs when a person seems to be employed...(Solved)
State the type of unemployment described below
i. Experienced where demand for goods and services is seasonal
ii. Occurs when a person seems to be employed but is mainly under
utilized
iii. Occurs when jobs are available but one does not have the required
qualifications
iv. Caused by Economic factors leading to poor performance of the
Economy
Date posted: May 22, 2019. Answers (1)
- Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.(Solved)
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
Date posted: May 22, 2019. Answers (1)
- The flow chart below shows the manufacture of a cleansing agent.
(i) Identify each of the substance D and L
(ii) Give one advantage of using this...(Solved)
The flow chart below shows the manufacture of a cleansing agent.
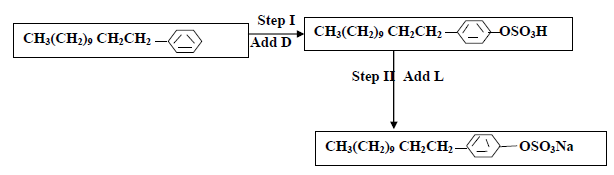
(i) Identify each of the substance D and L
(ii) Give one advantage of using this cleansing agent over ordinary soap
(iii) What is the effect of the above cleansing agent to the environment.
Date posted: May 22, 2019. Answers (1)
- The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that...(Solved)
The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that follow:
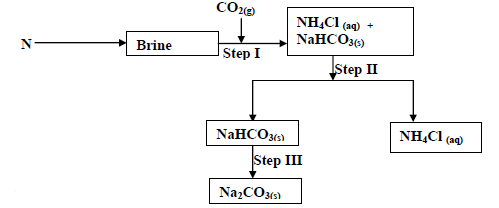
(a) Name substance N
(b) Name the process taking place in
(i) Step II
(ii) Step III
(c) Write an equation for the react producing sodium carbonate.
Date posted: May 22, 2019. Answers (1)
- Draw the structure of a sulphur molecule(Solved)
Draw the structure of a sulphur molecule
Date posted: May 22, 2019. Answers (1)
- The following is a list of pH values of some substance:(i) Strong acid (ii)Weak base(Solved)
The following is a list of pH values of some substance:

Identify:
(i) Strong acid
(ii)Weak base
Date posted: May 22, 2019. Answers (1)
- Distinguishing between weak and strong alkali(Solved)
Distinguishing between weak and strong alkali
Date posted: May 22, 2019. Answers (1)
- The structure below represents a polymer
(a) State the name of the polymer
(b) State one industrial use of the polymer(Solved)
The structure below represents a polymer
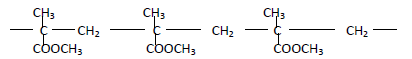
(a) State the name of the polymer
(b) State one industrial use of the polymer
Date posted: May 22, 2019. Answers (1)
- A dry gas X was passed over heated lead (II) oxide. A grey residue and a gas Y were formed.
The gas Y has no effect...(Solved)
A dry gas X was passed over heated lead (II) oxide. A grey residue and a gas Y were formed.
The gas Y has no effect on red litmus paper and does not support combustion. Identity:
(i) Gas X
(ii) Gas Y
Date posted: May 22, 2019. Answers (1)
- 20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.(Solved)
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
Date posted: May 22, 2019. Answers (1)
- Give one industrial application of solvent extraction(Solved)
Give one industrial application of solvent extraction
Date posted: May 22, 2019. Answers (1)