Dip the blue litmus paper in each solution. H2SO4 turns it red while K2CO3
and NaCl have no effect transfer the two unknown solutions to a test tube. Add
H2SO4 to each solutions solution with K2CO3 give vigorous effervescence
while solution of NaCl give no observable change.
sharon kalunda answered the question on May 22, 2019 at 13:56
- The scheme below shows a reaction sequence starting with solid N. study it and answer
the questions that follows.
a) Name the cation present in solid N....(Solved)
The scheme below shows a reaction sequence starting with solid N. study it and answer
the questions that follows.
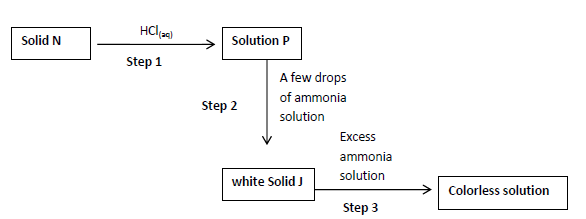
a) Name the cation present in solid N.
b) Write the formula of the complex ion in the colorless solution Q.
c) Write an ionic equation for the reaction in step 2.
Date posted: May 22, 2019. Answers (1)
- In an experiment, 40cm3 of 0.5M sulphuric (VI) acid was reacted with excess sodium
carbonate and the volume of cabon (IV) oxide produced recorded with...(Solved)
In an experiment, 40cm3 of 0.5M sulphuric (VI) acid was reacted with excess sodium
carbonate and the volume of cabon (IV) oxide produced recorded with time. In another
experiment, 40cm3 of 0.5M ethanioc acid was reacted with excess sodium carbonate and the
volume of carbon (IV) oxide produced recorded with time. On the grid below, sketch and
label the curves if the volumes of carbon (IV) oxide were plotted against time on the same
axis.
Date posted: May 22, 2019. Answers (1)
- What is meant by the term strong acid?(Solved)
What is meant by the term strong acid?
Date posted: May 22, 2019. Answers (1)
- Study the flow chart below showing the reaction involved in the preparation of sulphuric
(IV) acid and answer the questions that follow.(Solved)
Study the flow chart below showing the reaction involved in the preparation of sulphuric
(IV) acid and answer the questions that follow.
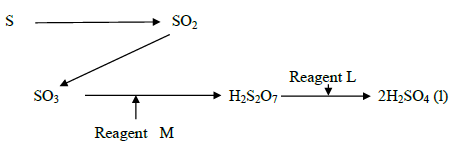
a) Name the reagents.
L and M
b) Write the equation for the reaction between reagent M and H2S2O7
Date posted: May 22, 2019. Answers (1)
- The set-up below was used to prepare Nitric(V) acid in the laboratory.
a) Name liquid T.
b) Write an equation for the reaction taking place in the...(Solved)
The set-up below was used to prepare Nitric(V) acid in the laboratory.
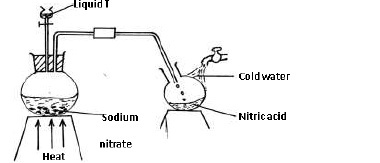
a) Name liquid T.
b) Write an equation for the reaction taking place in the flask.
c) State the reason why nitric (V) acid collected is brown in colour and explain how the
brown colour can be removed.
Date posted: May 22, 2019. Answers (1)
- a)Give the systematic names for the following compounds.
b) Describe one chemical process that can be used to distinguish between the
substances named in (a) above.(Solved)
a)Give the systematic names for the following compounds.

b) Describe one chemical process that can be used to distinguish between the
substances named in (a) above.
Date posted: May 22, 2019. Answers (1)
- The diagram below shows an industrial process that is used in extraction of sulphur.
a) What is the name given to the above industrial process?
b)...(Solved)
The diagram below shows an industrial process that is used in extraction of sulphur.
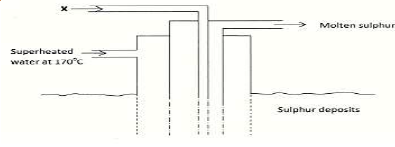
a) What is the name given to the above industrial process?
b) Identify substance X.
c) What is the role of super-heated water?
Date posted: May 22, 2019. Answers (1)
- The empirical formula of a compound is C2H5. When 11.6g of the compound was
allowed to evaporate; it occupied 4.8 dm3 at room temperature and pressure....(Solved)
The empirical formula of a compound is C2H5. When 11.6g of the compound was
allowed to evaporate; it occupied 4.8 dm3 at room temperature and pressure. What is
its molecular formula? (MGV = 24dm3)
Date posted: May 22, 2019. Answers (1)
- A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into...(Solved)
A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into a vacuum. An
identical amount of oxygen gas passes through the same aperture in 20 seconds.
Determine the molar mass of gas Z. (O=16, S=32).
Date posted: May 22, 2019. Answers (1)
- Write equations to show the effect of heat on each of the following.
(a) Sodium hydrogen carbonate.
(b) Silver nitrate
(c ) Sodium nitrate(Solved)
Write equations to show the effect of heat on each of the following.
(a) Sodium hydrogen carbonate.
(b) Silver nitrate
(c ) Sodium nitrate
Date posted: May 22, 2019. Answers (1)
- When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the...(Solved)
When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the white
precipitate dissolves to form a colorless solution.
a) Name the white precipitate.
b) Explain using chemical equations why the white precipitate dissolves in excess of
carbon(IV) oxide.
c) What will happen of the above colorless solution is boiled.
Date posted: May 22, 2019. Answers (1)
- When magnesium burns in air, it forms two products. When one of the products
dissolves in water, a colorless gas that turns red litmus paper blue...(Solved)
When magnesium burns in air, it forms two products. When one of the products
dissolves in water, a colorless gas that turns red litmus paper blue is formed.
a) Name the product that dissolves in water to produce a colorless gas.
b) Write an equation for the formation of the colorless gas.
c) State any one use of the colorless gas.
Date posted: May 22, 2019. Answers (1)
- The table below shows elements and their atomic numbers. The letters do not
represent the actual symbols of the elements.
a)From the given letters, select two elements...(Solved)
The table below shows elements and their atomic numbers. The letters do not
represent the actual symbols of the elements.
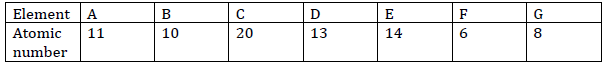
a)From the given letters, select two elements with the same chemical properties.
b) Write the formula of the compound formed when element E reacts with element G.
c) Identify the most stable element and give a reason for your answer.
Date posted: May 22, 2019. Answers (1)
- A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
Identify with reasons two mistakes in the above set-up.(Solved)
A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
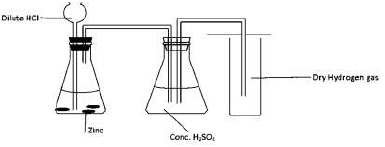
Identify with reasons two mistakes in the above set-up.
Date posted: May 22, 2019. Answers (1)
- The following chromatogram shows the results obtained after separating substances P
and T.(Solved)
The following chromatogram shows the results obtained after separating substances P
and T.
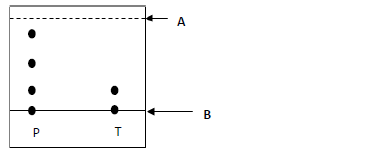
a) Name lines;
A
B
b) Name the possible solvent that can be used in the above process.
c) Which of the two substances is pure?
Date posted: May 22, 2019. Answers (1)
- In an experiment to separate a mixture of two immiscible liquids A and B, a form four
student set the apparatus as shown below.
a) Name the...(Solved)
In an experiment to separate a mixture of two immiscible liquids A and B, a form four
student set the apparatus as shown below.
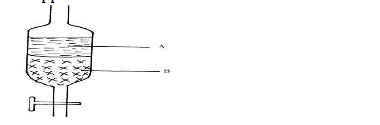
a) Name the above apparatus.
b) Which liquid is denser?
c) Name one other method that can be used to separate the above mixture.
Date posted: May 22, 2019. Answers (1)
- The diagram below shows a Bunsen burner which is used as a source of heat in the
laboratory.(Solved)
The diagram below shows a Bunsen burner which is used as a source of heat in the
laboratory.
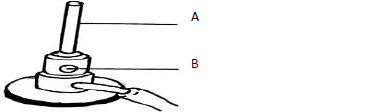
Name the parts labeled: (
A
B
Date posted: May 22, 2019. Answers (1)
- State the type of unemployment described below
i. Experienced where demand for goods and services is seasonal
ii. Occurs when a person seems to be employed...(Solved)
State the type of unemployment described below
i. Experienced where demand for goods and services is seasonal
ii. Occurs when a person seems to be employed but is mainly under
utilized
iii. Occurs when jobs are available but one does not have the required
qualifications
iv. Caused by Economic factors leading to poor performance of the
Economy
Date posted: May 22, 2019. Answers (1)
- Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.(Solved)
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
Date posted: May 22, 2019. Answers (1)
- The flow chart below shows the manufacture of a cleansing agent.
(i) Identify each of the substance D and L
(ii) Give one advantage of using this...(Solved)
The flow chart below shows the manufacture of a cleansing agent.
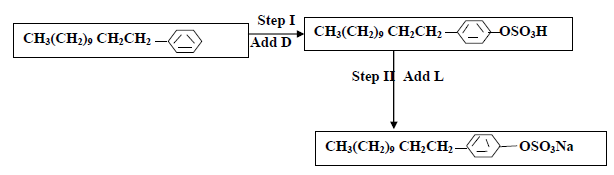
(i) Identify each of the substance D and L
(ii) Give one advantage of using this cleansing agent over ordinary soap
(iii) What is the effect of the above cleansing agent to the environment.
Date posted: May 22, 2019. Answers (1)