a) Amphoteric oxides.
b) Zinc oxide, aluminum oxide, lead(II)oxide.
sharon kalunda answered the question on May 22, 2019 at 14:15
-
The solubility of copper (II) sulphate is 55g/100g of water at 75oC and 19g/100g of
water at 15oC. What mass of crystals would be deposited, if...
(Solved)
The solubility of copper (II) sulphate is 55g/100g of water at 75oC and 19g/100g of
water at 15oC. What mass of crystals would be deposited, if 150g of a saturated solution
is cooled from 75oC to 15oC.
Date posted:
May 22, 2019
.
Answers (1)
-
A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into...
(Solved)
A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into a vacuum. An
identical amount of oxygen gas passes through the same aperture in 20 seconds.
Determine the molar mass of gas Z. (O=16, S=32).
Date posted:
May 22, 2019
.
Answers (1)
-
When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the...
(Solved)
When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the white
precipitate dissolves to form a colorless solution.
a) Name the white precipitate.
b) Explain using chemical equations why the white precipitate dissolves in excess of
carbon(IV) oxide.
c) What will happen of the above colorless solution is boiled.
Date posted:
May 22, 2019
.
Answers (1)
-
A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
Identify with reasons two mistakes in the above set-up.
(Solved)
A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
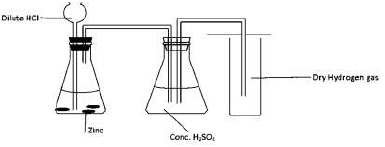
Identify with reasons two mistakes in the above set-up.
Date posted:
May 22, 2019
.
Answers (1)
-
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
(Solved)
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
Date posted:
May 22, 2019
.
Answers (1)
-
The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that...
(Solved)
The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that follow:
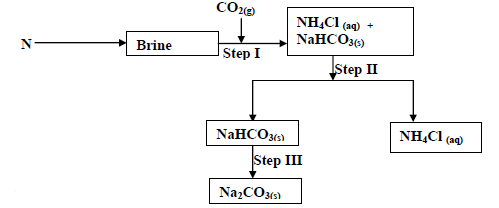
(a) Name substance N
(b) Name the process taking place in
(i) Step II
(ii) Step III
(c) Write an equation for the react producing sodium carbonate.
Date posted:
May 22, 2019
.
Answers (1)
-
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
(Solved)
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
Date posted:
May 22, 2019
.
Answers (1)
-
In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another containing 70cm3 of hydrogen sulphide gas. The two gases reacted according...
(Solved)
In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another
containing 70cm3 of hydrogen sulphide gas. The two gases reacted according to the equation
below to form 80cm3 of hydrogen chloride gas.
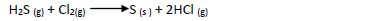
(a) Using oxidation number identify the oxidizing agent
(b) Calculate the percentage yield of hydrogen chloride gas
Date posted:
May 22, 2019
.
Answers (1)
-
Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution...
(Solved)
Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution of concentrated sodium chloride for 30 minutes.
(Na = 23, Cl = 35.5, Faraday constant = 96500, M.G V = 22.4dm3)
Date posted:
May 22, 2019
.
Answers (1)
-
Describe how the mixture of Ammonium chloride, sodium chloride and lead II chloride can be separated if all the components of the mixture are to...
(Solved)
Describe how the mixture of Ammonium chloride, sodium chloride and lead II chloride can be separated if all the components of the mixture are to be recovered.
Date posted:
May 22, 2019
.
Answers (1)
-
The diagram below represents an arrangement for a large scale manufacture of ethanol for domestic consumption.
(i) Name the process by which ethanol is obtained from...
(Solved)
The diagram below represents an arrangement for a large scale manufacture of ethanol for domestic consumption.
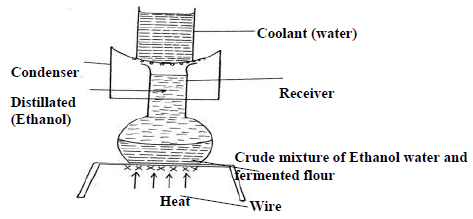
(i) Name the process by which ethanol is obtained from the crude oil.
(ii) Suggest two reasons why water is a coolant in this process.
(iii) Why is it possible to separate ethanol from the mixture by this process.
Date posted:
May 22, 2019
.
Answers (1)
-
In an experiment, a few drops of concentrated nitric (IV)acid were added to aqueous Iron (II)
sulphate in a test tube. excess sodium hydroxide solution was...
(Solved)
In an experiment, a few drops of concentrated nitric (IV)acid were added to aqueous Iron (II)
sulphate in a test tube. excess sodium hydroxide solution was then added to the mixture.
(a) State the observations that were made when:
(i) Concentrated nitric (V) acid was added to aqueous Iron (II) sulphate.
(ii) Excess sodium hydroxide was added to the mixture.
(b) Write an ionic equation for the reaction that occurred in a(ii) above.
Date posted:
May 22, 2019
.
Answers (1)
-
The diagram below shows a set-up of apparatus used to separate miscible liquids.
(a) Name the parts labelled A and B
(b) State the function of...
(Solved)
The diagram below shows a set-up of apparatus used to separate miscible liquids.
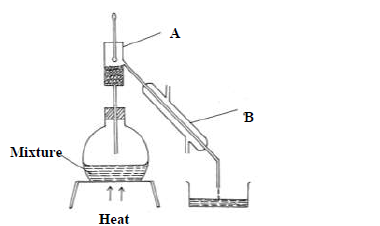
(a) Name the parts labelled A and B
(b) State the function of the part labeled A.
(c) State the property of the mixture that makes it suitable to be separated by the
method above.
Date posted:
May 22, 2019
.
Answers (1)
-
A volume of 80cm3 of a mixture of propane (C3H8) and oxygen were ignited in an experiment.The products were cooled and passed through an aqueous...
(Solved)
A volume of 80cm3 of a mixture of propane (C3H8) and oxygen were ignited in an experiment.The products were cooled and passed through an aqueous sodium hydroxide. The final volume
was reduced by 30cm3
a) Write the equation for the combustion of propane
b) Determine the volume of;
i) The component of the original mixture
ii) Residual oxygen
Date posted:
May 21, 2019
.
Answers (1)
-
75g of a saturated solution contains 30g of salt calculate
a) The solubility of the salt
b) The percentage of the salt in the saturated solution
(Solved)
75g of a saturated solution contains 30g of salt calculate
a) The solubility of the salt
b) The percentage of the salt in the saturated solution
Date posted:
May 21, 2019
.
Answers (1)
-
Draw a dot (.) and cross (x) diagram to show bonding in:-
(i) Ammonium ion (NH4)
(ii) Silane (SiH4)
(N=14...
(Solved)
Draw a dot (.) and cross (x) diagram to show bonding in:-
(i) Ammonium ion (NH4)
(ii) Silane (SiH4)
(N=14 H=1 Si=14)
Date posted:
May 21, 2019
.
Answers (1)
-
Explain why graphite conducts electricity while diamond does not.
(Solved)
Explain why graphite conducts electricity while diamond does not.
Date posted:
May 21, 2019
.
Answers (1)
-
When 31.2g of hydrated. Aluminium oxide ( Al2O3XH2O) was heated to a constant mass of 20.6g of Aluminium oxide ( Al2O3) was obtained. Determine the value...
(Solved)
When 31.2g of hydrated. Aluminium oxide ( Al2O3XH2O) was heated to a constant
mass of 20.6g of Aluminium oxide ( Al2O3) was obtained. Determine the value of x in
hydrated oxide.(Al= 27.0, O=16.0, H=1.0)
Date posted:
May 21, 2019
.
Answers (1)
-
The flow chart below shows industrial extraction Aluminium metal. Study it and answer the questions that follow.
(i) Explain how process T is carried out.
(ii)...
(Solved)
The flow chart below shows industrial extraction Aluminium metal. Study it and answer the questions that follow.

(i) Explain how process T is carried out.
(ii) Name residue P, give a reason.
(iii) Explain why it is necessary to heat Aluminium oxide in presence of cryolite before
electrolysis is carried out.
Date posted:
May 21, 2019
.
Answers (1)
-
Describe how sulphuric acid is manufactured from sulphur (VI) oxide.
(Solved)
Describe how sulphuric acid is manufactured from sulphur (VI) oxide.
Date posted:
May 21, 2019
.
Answers (1)