- x grammes of a radioactive isotope decayed to 5 grammes in 100 days. The half-life of the isotope is 25 days.
a) Define half life.
b)...(Solved)
x grammes of a radioactive isotope decayed to 5 grammes in 100 days. The half-life of the isotope is 25 days.
a) Define half life.
b) Calculate the initial mass x of the radioactive isotope.
Date posted: May 23, 2019. Answers (1)
- Study the scheme below and answer the questions that follow.
a) Write the formular of the cation present in solution D.
b) What property of chlorine is...(Solved)
Study the scheme below and answer the questions that follow.

a) Write the formular of the cation present in solution D.
b) What property of chlorine is shown in step 1.
Date posted: May 23, 2019. Answers (1)
- In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form...(Solved)
In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form lather. with 100cm³ of each sample of water before and after heating / boiling.

a) Which water sample is likely to be soft? Explain.
b) Explain the change in the volume of soap solution used in sample C.
Date posted: May 23, 2019. Answers (1)
- The table below gives some properties of gases X and Y. Study it and answer the questions that follow.
a) Describe how you would obtain a...(Solved)
The table below gives some properties of gases X and Y. Study it and answer the questions that follow.

a) Describe how you would obtain a sample of gas Y from a mixture of gases X and Y.
b) Suggest a possible identity of gas X. Give a reason for your answer.
Date posted: May 23, 2019. Answers (1)
- Give two reasons why duralumin is preferred to aluminium in making aeroplane parts.(Solved)
Give two reasons why duralumin is preferred to aluminium in making aeroplane parts.
Date posted: May 23, 2019. Answers (1)
- The table below gives three experiments on the reaction of excess sulphuric (VI) acid and 0.5g Zinc done under different conditions. In each case the...(Solved)
The table below gives three experiments on the reaction of excess sulphuric (VI) acid and 0.5g Zinc done under different conditions. In each case the volume of gas liberated was recorded at different time intervals.
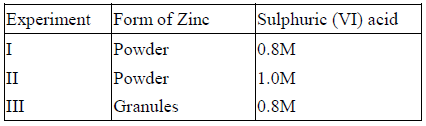
On the axes below, draw and label the three curves that would be obtained from the results above.
Date posted: May 23, 2019. Answers (1)
- Use the bond energies given below to calculate the heat of reaction for;(Solved)
Use the bond energies given below to calculate the heat of reaction for;

Date posted: May 23, 2019. Answers (1)
- Study the diagram below then use it to answer the questions that follow.
a) Draw the wooden splint at the end of the experiment. If it...(Solved)
Study the diagram below then use it to answer the questions that follow.

a) Draw the wooden splint at the end of the experiment. If it was slipped then removed.
b) Explain the appearance of the wooden splint in (a) above.
Date posted: May 23, 2019. Answers (1)
- Which is the chemical test to differentiate between alkenes and alkynes?(Solved)
Which is the chemical test to differentiate between alkenes and alkynes?
Date posted: May 22, 2019. Answers (1)
- Use the table of logarithms to evaluate. (Solved)
Use the table of logarithms to evaluate.

Date posted: May 22, 2019. Answers (1)
- The diagram below shows acidic and basic oxides fit in a general family of oxides.
a) State the name given to the type of oxides that...(Solved)
The diagram below shows acidic and basic oxides fit in a general family of oxides.

a) State the name given to the type of oxides that would be placed in the shaded region.
b) Name two oxides that could be placed on the shaded region.
Date posted: May 22, 2019. Answers (1)
- Substances X and Y consists of molecules X2 and Y2 respectively. When the two
elements react, they form a molecule of XY. The X-X bonds are...(Solved)
Substances X and Y consists of molecules X2 and Y2 respectively. When the two
elements react, they form a molecule of XY. The X-X bonds are as strong as Y-Y bonds.
But the X-Y bond is stronger than both X-X and Y-Y bonds.

a) Is the above reaction exothermic or endothermic? Give a reason for your answer
b) Draw an energy level diagram for the reaction in (a) above.
Date posted: May 22, 2019. Answers (1)
- Using well labeled diagrams, explain how water hardness can be removed by ion
exchange method.(Solved)
Using well labeled diagrams, explain how water hardness can be removed by ion
exchange method.
Date posted: May 22, 2019. Answers (1)
- The solubility of copper (II) sulphate is 55g/100g of water at 75oC and 19g/100g of
water at 15oC. What mass of crystals would be deposited, if...(Solved)
The solubility of copper (II) sulphate is 55g/100g of water at 75oC and 19g/100g of
water at 15oC. What mass of crystals would be deposited, if 150g of a saturated solution
is cooled from 75oC to 15oC.
Date posted: May 22, 2019. Answers (1)
- Some bond energies are given below;
Calculate the energy change for the reaction below.(Solved)
Some bond energies are given below;
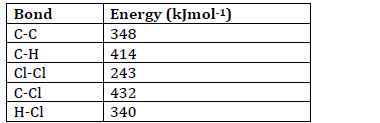
Calculate the energy change for the reaction below.
Date posted: May 22, 2019. Answers (1)
- Reagent bottles labelled H2SO4 solution, K2CO3 solution and NaCl solution had labels
accidentally removed. A packet of blue litmus paper is lying near a long with...(Solved)
Reagent bottles labelled H2SO4 solution, K2CO3 solution and NaCl solution had labels
accidentally removed. A packet of blue litmus paper is lying near a long with a rack of test-tubes, without using any other material explain how you would go about labeling the bottles correctly.
Date posted: May 22, 2019. Answers (1)
- The scheme below shows a reaction sequence starting with solid N. study it and answer
the questions that follows.
a) Name the cation present in solid N....(Solved)
The scheme below shows a reaction sequence starting with solid N. study it and answer
the questions that follows.
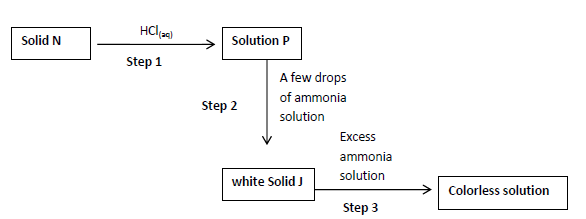
a) Name the cation present in solid N.
b) Write the formula of the complex ion in the colorless solution Q.
c) Write an ionic equation for the reaction in step 2.
Date posted: May 22, 2019. Answers (1)
- In an experiment, 40cm3 of 0.5M sulphuric (VI) acid was reacted with excess sodium
carbonate and the volume of cabon (IV) oxide produced recorded with...(Solved)
In an experiment, 40cm3 of 0.5M sulphuric (VI) acid was reacted with excess sodium
carbonate and the volume of cabon (IV) oxide produced recorded with time. In another
experiment, 40cm3 of 0.5M ethanioc acid was reacted with excess sodium carbonate and the
volume of carbon (IV) oxide produced recorded with time. On the grid below, sketch and
label the curves if the volumes of carbon (IV) oxide were plotted against time on the same
axis.
Date posted: May 22, 2019. Answers (1)
- What is meant by the term strong acid?(Solved)
What is meant by the term strong acid?
Date posted: May 22, 2019. Answers (1)
- Study the flow chart below showing the reaction involved in the preparation of sulphuric
(IV) acid and answer the questions that follow.(Solved)
Study the flow chart below showing the reaction involved in the preparation of sulphuric
(IV) acid and answer the questions that follow.
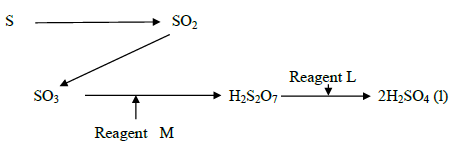
a) Name the reagents.
L and M
b) Write the equation for the reaction between reagent M and H2S2O7
Date posted: May 22, 2019. Answers (1)