Solution M - increased in volume because solution M is hypertonic to solution K; therefore solution K moved into visking tubing by osmosis;
Solution L - remained the same volume because it was isotonic to solution K, osmosis did not occur ;
sharon kalunda answered the question on May 23, 2019 at 12:26
- Study the diagram below and answer the questions that follow.
a) Identify the diagram above.
b) State one reason for your answer in (a) above.
c)...(Solved)
Study the diagram below and answer the questions that follow.
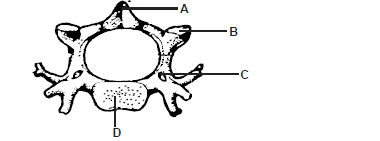
a) Identify the diagram above.
b) State one reason for your answer in (a) above.
c) Name the parts labelled A and B.
Date posted: May 23, 2019. Answers (1)
- The set-up below shows an experiment to investigate photosynthesis.
a) What gas was collected in the test-tube.
b) What two environmental conditions are necessary for the...(Solved)
The set-up below shows an experiment to investigate photosynthesis.

a) What gas was collected in the test-tube.
b) What two environmental conditions are necessary for the experiment?
c) What was the role of sodium hydrogen carbonate in the experiment.
Date posted: May 23, 2019. Answers (1)
- Give a reason for each of the following:
a) Wind pollinated flowers produce large quantities of pollen grains.
b) Flowers of certain plants show heterostyly.(Solved)
Give a reason for each of the following:
a) Wind pollinated flowers produce large quantities of pollen grains.
b) Flowers of certain plants show heterostyly.
Date posted: May 23, 2019. Answers (1)
- Name the part of the brain responsible for:
a) Maintaining balance and posture of the body.
b) Controls heart, breathing and involuntary responses.(Solved)
Name the part of the brain responsible for:
a) Maintaining balance and posture of the body.
b) Controls heart, breathing and involuntary responses.
Date posted: May 23, 2019. Answers (1)
- Explain why protein is absent in urine of a normal person.(Solved)
Explain why protein is absent in urine of a normal person.
Date posted: May 23, 2019. Answers (1)
- Name the types of joints formed by each of the following pairs of bones.
i) Axis and Atlas.
ii) Humerus with clavicle and scapula.
iii) Tibia and...(Solved)
Name the types of joints formed by each of the following pairs of bones.
i) Axis and Atlas.
ii) Humerus with clavicle and scapula.
iii) Tibia and fibula with femur.
Date posted: May 23, 2019. Answers (1)
- Why is pyramid of biomass a better method of representing ecological relationships in habitats?(Solved)
Why is pyramid of biomass a better method of representing ecological relationships in habitats?
Date posted: May 23, 2019. Answers (1)
- Name a respiratory substrate usually available for energy release during starvation.(Solved)
Name a respiratory substrate usually available for energy release during starvation.
Date posted: May 23, 2019. Answers (1)
- Explain why a cross-circuit athlete pants heavily after sprint race.(Solved)
Explain why a cross-circuit athlete pants heavily after sprint race.
Date posted: May 23, 2019. Answers (1)
- In a family with four children, the father had blood group A while the mother had blood group B. One of the children had
blood group...(Solved)
In a family with four children, the father had blood group A while the mother had blood group B. One of the children had
blood group O.
a) What are the genotypes of the parents
b) What was the genotype of the child with blood group O?
Date posted: May 23, 2019. Answers (1)
- A potato cylinder measuring 100 mm was placed in a concentrated salt solution for 30 minutes. Describe its texture and
appearance after 30 minutes.(Solved)
A potato cylinder measuring 100 mm was placed in a concentrated salt solution for 30 minutes. Describe its texture and
appearance after 30 minutes.
Date posted: May 23, 2019. Answers (1)
- State two disadvantages of fossil records as an evidence of evolution.(Solved)
State two disadvantages of fossil records as an evidence of evolution.
Date posted: May 23, 2019. Answers (1)
- State the importance of the following processes to living organisms.
a) Locomotion.
b) Irritability(Solved)
State the importance of the following processes to living organisms.
a) Locomotion.
b) Irritability
Date posted: May 23, 2019. Answers (1)
- Explain why herbivores have large eyes on their sides?(Solved)
Explain why herbivores have large eyes on their sides?
Date posted: May 22, 2019. Answers (1)
- The following micrographs show images taken from a transverse section of a various stems by
a light microscope. Analyze them closely and use them to answer...(Solved)
The following micrographs show images taken from a transverse section of a various stems by
a light microscope. Analyze them closely and use them to answer questions that follow.

a)On the diagram, label part A, B and C
b) Explain the adaptation of the parts C and D to their functions
c) Identify five differences between cross section T and Q.
d) Explain how part B facilitates the process of secondary growth
Date posted: May 22, 2019. Answers (1)
- The Diagram below shows two organisms (R and S) belonging to the same phylum
(a) Name the class in which the organisms shown above belong.
i)...(Solved)
The Diagram below shows two organisms (R and S) belonging to the same phylum

(a) Name the class in which the organisms shown above belong.
i) Organism R
ii) Organism S
b) Other than presence of exoskeleton, list two observable similarities between the two organisms
c) List two observable differences between the two organisms
d) Explain how the organism labelled R is adapted to safeguard itself from the predator
e) (i) Name the gaseous exchange system exhibited by organism S
ii) State the respiratory surface used by organism S
Date posted: May 22, 2019. Answers (1)
- Describe the process of exhalation in mammals.(Solved)
Describe the process of exhalation in mammals.
Date posted: May 22, 2019. Answers (1)
- Discuss/Describe gaseous exchange in alveolus.(Solved)
Describe gaseous exchange in alveolus.
Date posted: May 22, 2019. Answers (1)
- An experiment was set up to demonstrate the necessity of carbon (IV) oxide for
photosynthesis in a certain green plant as shown below. The plant was...(Solved)
An experiment was set up to demonstrate the necessity of carbon (IV) oxide for
photosynthesis in a certain green plant as shown below. The plant was first kept
darkness for 48 hours before the experiment.

a), Why was the plant kept in darkness for 48 hours before the start of this experiment.
b), What was the role of sodium hydroxide?
ci)What was the results shown by the leaf in the flask when it was tested for presence of starch after
the set up was exposed to light for a day?.
ii), Give reasons for your answer in (c) I above
d. Suggest a control for this experiment.
e), Name other two limiting factors in this experiment.
Date posted: May 22, 2019. Answers (1)
- Name two harmones that bring about rapid cell division in plants(Solved)
Name two harmones that bring about rapid cell division in plants
Date posted: May 22, 2019. Answers (1)