-
Calculate the number of molecules of water of crystallization in oxalic acid crystals, H2C2O4.nH2O given that 5g of the
crystals were made upto 250cm³ of this...
(Solved)
Calculate the number of molecules of water of crystallization in oxalic acid crystals, H2C2O4.nH2O given that 5g of the
crystals were made upto 250cm³ of this solution. 25.0cm³ of this solution required 15.9cm³ of 0.5M sodium hydroxide to
neutralise it (H=1, C=12, O=16).
Date posted:
May 23, 2019
.
Answers (1)
-
Nylon 6, 6 is a condensation polymer whose structure is as follows.
Draw the structures of the monomers in nylon 6, 6
(Solved)
Nylon 6, 6 is a condensation polymer whose structure is as follows.

Draw the structures of the monomers in nylon 6, 6
Date posted:
May 23, 2019
.
Answers (1)
-
Magnesium reacts as shown below.
a) Identify gas X.
b) Between wet sand and magnesium ribbon, which one should be heated first? Explain.
(Solved)
Magnesium reacts as shown below.


a) Identify gas X.
b) Between wet sand and magnesium ribbon, which one should be heated first? Explain.
Date posted:
May 23, 2019
.
Answers (1)
-
The diagram below was used to electrolyse magnesium sulphate solution
a) Write half equation at electrode. P,Q
b) State what happens to the concentration of the...
(Solved)
The diagram below was used to electrolyse magnesium sulphate solution
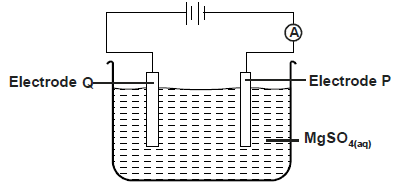
a) Write half equation at electrode. P,Q
b) State what happens to the concentration of the electrolyte after electrolysis process.
Date posted:
May 23, 2019
.
Answers (1)
-
Use the diagram below to answer the questions that follow.
a) Why is sodium hydroxide solution preferred to water in the above set-up.
b) What modification should...
(Solved)
Use the diagram below to answer the questions that follow.
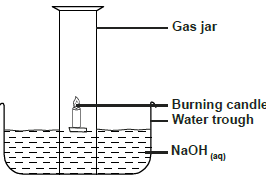
a) Why is sodium hydroxide solution preferred to water in the above set-up.
b) What modification should be made to the above set-up if percentage of oxygen used in air should be determined
c) Name the main component of air not used in the above set-up.
Date posted:
May 23, 2019
.
Answers (1)
-
0.63g of lead powder were dissolved in excess nitric (V) acid to form lead (II) nitrate solution. All the lead (II) nitrate was
then reacted with...
(Solved)
0.63g of lead powder were dissolved in excess nitric (V) acid to form lead (II) nitrate solution. All the lead (II) nitrate was
then reacted with sodium sulphate solution.
a) Write an ionic equation for the reaction between sodium sulphate solution and lead (II) nitrate solution.
b) Determine the mass of the lead salt formed in the reaction in (a) above
(Pb = 207, S = 32, O = 16)
Date posted:
May 23, 2019
.
Answers (1)
-
Study the diagram below then use it to answer the questions that follow.
a) Draw the wooden splint at the end of the experiment. If it...
(Solved)
Study the diagram below then use it to answer the questions that follow.

a) Draw the wooden splint at the end of the experiment. If it was slipped then removed.
b) Explain the appearance of the wooden splint in (a) above.
Date posted:
May 23, 2019
.
Answers (1)
-
The diagram below shows acidic and basic oxides fit in a general family of oxides.
a) State the name given to the type of oxides that...
(Solved)
The diagram below shows acidic and basic oxides fit in a general family of oxides.

a) State the name given to the type of oxides that would be placed in the shaded region.
b) Name two oxides that could be placed on the shaded region.
Date posted:
May 22, 2019
.
Answers (1)
-
The solubility of copper (II) sulphate is 55g/100g of water at 75oC and 19g/100g of
water at 15oC. What mass of crystals would be deposited, if...
(Solved)
The solubility of copper (II) sulphate is 55g/100g of water at 75oC and 19g/100g of
water at 15oC. What mass of crystals would be deposited, if 150g of a saturated solution
is cooled from 75oC to 15oC.
Date posted:
May 22, 2019
.
Answers (1)
-
A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into...
(Solved)
A sample of unknown gas Z was shown by analysis to contain sulphur and oxygen.
The gas requires 28.3 seconds to diffuse through an aperture into a vacuum. An
identical amount of oxygen gas passes through the same aperture in 20 seconds.
Determine the molar mass of gas Z. (O=16, S=32).
Date posted:
May 22, 2019
.
Answers (1)
-
When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the...
(Solved)
When small amount of carbon (IV) oxide is passed through lime water, a white
precipitate is formed. When excess carbon (IV) oxide is bubbled through, the white
precipitate dissolves to form a colorless solution.
a) Name the white precipitate.
b) Explain using chemical equations why the white precipitate dissolves in excess of
carbon(IV) oxide.
c) What will happen of the above colorless solution is boiled.
Date posted:
May 22, 2019
.
Answers (1)
-
A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
Identify with reasons two mistakes in the above set-up.
(Solved)
A form four student arranged the apparatus as shown below with the aim of collecting
dry hydrogen gas.
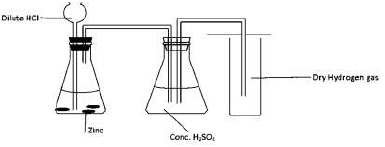
Identify with reasons two mistakes in the above set-up.
Date posted:
May 22, 2019
.
Answers (1)
-
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
(Solved)
Explain why cooking pots made of aluminium do not corrode easily when exposed to
air.
Date posted:
May 22, 2019
.
Answers (1)
-
The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that...
(Solved)
The flow chart below shows some of the stages in the manufacture of sodium carbonate by the
solvay process. Use it to answer the questions that follow:
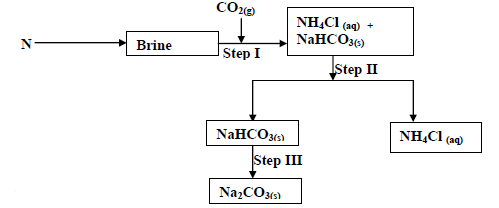
(a) Name substance N
(b) Name the process taking place in
(i) Step II
(ii) Step III
(c) Write an equation for the react producing sodium carbonate.
Date posted:
May 22, 2019
.
Answers (1)
-
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
(Solved)
20cm3 of sodium hydroxide solution containing 8.0g/dm3 were required for complete
neutralization of 0.18g of a dibasic acid H2X.
Calculate the relative molecular mass of the acid.
Date posted:
May 22, 2019
.
Answers (1)
-
In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another containing 70cm3 of hydrogen sulphide gas. The two gases reacted according...
(Solved)
In an experiment a gas jar containing 70cm3 of chlorine gas was inverted over another
containing 70cm3 of hydrogen sulphide gas. The two gases reacted according to the equation
below to form 80cm3 of hydrogen chloride gas.
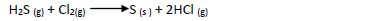
(a) Using oxidation number identify the oxidizing agent
(b) Calculate the percentage yield of hydrogen chloride gas
Date posted:
May 22, 2019
.
Answers (1)
-
Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution...
(Solved)
Calculate the volume of chlorine gas in cm3 (measured at s.t.p) that is formed when a current of 0.9 A is passed through a solution of concentrated sodium chloride for 30 minutes.
(Na = 23, Cl = 35.5, Faraday constant = 96500, M.G V = 22.4dm3)
Date posted:
May 22, 2019
.
Answers (1)
-
Describe how the mixture of Ammonium chloride, sodium chloride and lead II chloride can be separated if all the components of the mixture are to...
(Solved)
Describe how the mixture of Ammonium chloride, sodium chloride and lead II chloride can be separated if all the components of the mixture are to be recovered.
Date posted:
May 22, 2019
.
Answers (1)
-
The diagram below represents an arrangement for a large scale manufacture of ethanol for domestic consumption.
(i) Name the process by which ethanol is obtained from...
(Solved)
The diagram below represents an arrangement for a large scale manufacture of ethanol for domestic consumption.
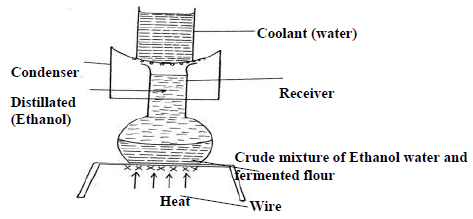
(i) Name the process by which ethanol is obtained from the crude oil.
(ii) Suggest two reasons why water is a coolant in this process.
(iii) Why is it possible to separate ethanol from the mixture by this process.
Date posted:
May 22, 2019
.
Answers (1)
-
In an experiment, a few drops of concentrated nitric (IV)acid were added to aqueous Iron (II)
sulphate in a test tube. excess sodium hydroxide solution was...
(Solved)
In an experiment, a few drops of concentrated nitric (IV)acid were added to aqueous Iron (II)
sulphate in a test tube. excess sodium hydroxide solution was then added to the mixture.
(a) State the observations that were made when:
(i) Concentrated nitric (V) acid was added to aqueous Iron (II) sulphate.
(ii) Excess sodium hydroxide was added to the mixture.
(b) Write an ionic equation for the reaction that occurred in a(ii) above.
Date posted:
May 22, 2019
.
Answers (1)