2 moles Li ® (2 × 24)dm³
Kavungya answered the question on May 24, 2019 at 08:00
- When sulphur is heated in a boiling tube in the absence of air, the yellow crystals melts into a golden yellow mobile liquid at
113°C. The...(Solved)
When sulphur is heated in a boiling tube in the absence of air, the yellow crystals melts into a golden yellow mobile liquid at
113°C. The liquid changes at 180°C into a dark brown liquid that is very viscous. More heating at 400°C produces a brown
less viscous liquid.
a) Draw the molecular structure of sulphur in the yellow liquid.
b) Explain why the molten liquid becomes viscous.
Date posted: May 23, 2019. Answers (1)
- Use dots (•) and cross (×) diagrams to draw bond in:
a) Al2Cl6 (Al = 13, Cl=17)
b) Al2O3 (Al = 13, O = 8)(Solved)
Use dots (•) and cross (×) diagrams to draw bond in:
a) Al2Cl6 (Al = 13, Cl=17)
b) Al2O3 (Al = 13, O = 8)
Date posted: May 23, 2019. Answers (1)
- Calculate the number of molecules of water of crystallization in oxalic acid crystals, H2C2O4.nH2O given that 5g of the
crystals were made upto 250cm³ of this...(Solved)
Calculate the number of molecules of water of crystallization in oxalic acid crystals, H2C2O4.nH2O given that 5g of the
crystals were made upto 250cm³ of this solution. 25.0cm³ of this solution required 15.9cm³ of 0.5M sodium hydroxide to
neutralise it (H=1, C=12, O=16).
Date posted: May 23, 2019. Answers (1)
- Nylon 6, 6 is a condensation polymer whose structure is as follows.
Draw the structures of the monomers in nylon 6, 6(Solved)
Nylon 6, 6 is a condensation polymer whose structure is as follows.

Draw the structures of the monomers in nylon 6, 6
Date posted: May 23, 2019. Answers (1)
- Magnesium reacts as shown below.
a) Identify gas X.
b) Between wet sand and magnesium ribbon, which one should be heated first? Explain.(Solved)
Magnesium reacts as shown below.


a) Identify gas X.
b) Between wet sand and magnesium ribbon, which one should be heated first? Explain.
Date posted: May 23, 2019. Answers (1)
- The diagram below was used to electrolyse magnesium sulphate solution
a) Write half equation at electrode. P,Q
b) State what happens to the concentration of the...(Solved)
The diagram below was used to electrolyse magnesium sulphate solution
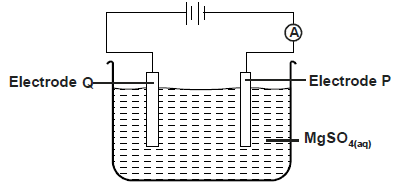
a) Write half equation at electrode. P,Q
b) State what happens to the concentration of the electrolyte after electrolysis process.
Date posted: May 23, 2019. Answers (1)
- Use the diagram below to answer the questions that follow.
a) Why is sodium hydroxide solution preferred to water in the above set-up.
b) What modification should...(Solved)
Use the diagram below to answer the questions that follow.
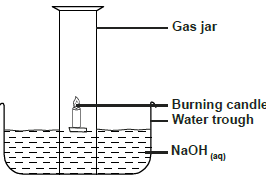
a) Why is sodium hydroxide solution preferred to water in the above set-up.
b) What modification should be made to the above set-up if percentage of oxygen used in air should be determined
c) Name the main component of air not used in the above set-up.
Date posted: May 23, 2019. Answers (1)
- 0.63g of lead powder were dissolved in excess nitric (V) acid to form lead (II) nitrate solution. All the lead (II) nitrate was
then reacted with...(Solved)
0.63g of lead powder were dissolved in excess nitric (V) acid to form lead (II) nitrate solution. All the lead (II) nitrate was
then reacted with sodium sulphate solution.
a) Write an ionic equation for the reaction between sodium sulphate solution and lead (II) nitrate solution.
b) Determine the mass of the lead salt formed in the reaction in (a) above
(Pb = 207, S = 32, O = 16)
Date posted: May 23, 2019. Answers (1)
- x grammes of a radioactive isotope decayed to 5 grammes in 100 days. The half-life of the isotope is 25 days.
a) Define half life.
b)...(Solved)
x grammes of a radioactive isotope decayed to 5 grammes in 100 days. The half-life of the isotope is 25 days.
a) Define half life.
b) Calculate the initial mass x of the radioactive isotope.
Date posted: May 23, 2019. Answers (1)
- Study the scheme below and answer the questions that follow.
a) Write the formular of the cation present in solution D.
b) What property of chlorine is...(Solved)
Study the scheme below and answer the questions that follow.

a) Write the formular of the cation present in solution D.
b) What property of chlorine is shown in step 1.
Date posted: May 23, 2019. Answers (1)
- In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form...(Solved)
In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form lather. with 100cm³ of each sample of water before and after heating / boiling.

a) Which water sample is likely to be soft? Explain.
b) Explain the change in the volume of soap solution used in sample C.
Date posted: May 23, 2019. Answers (1)
- The table below gives some properties of gases X and Y. Study it and answer the questions that follow.
a) Describe how you would obtain a...(Solved)
The table below gives some properties of gases X and Y. Study it and answer the questions that follow.

a) Describe how you would obtain a sample of gas Y from a mixture of gases X and Y.
b) Suggest a possible identity of gas X. Give a reason for your answer.
Date posted: May 23, 2019. Answers (1)
- Give two reasons why duralumin is preferred to aluminium in making aeroplane parts.(Solved)
Give two reasons why duralumin is preferred to aluminium in making aeroplane parts.
Date posted: May 23, 2019. Answers (1)
- The table below gives three experiments on the reaction of excess sulphuric (VI) acid and 0.5g Zinc done under different conditions. In each case the...(Solved)
The table below gives three experiments on the reaction of excess sulphuric (VI) acid and 0.5g Zinc done under different conditions. In each case the volume of gas liberated was recorded at different time intervals.
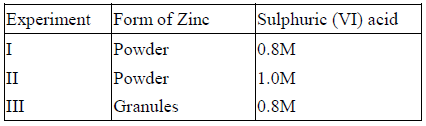
On the axes below, draw and label the three curves that would be obtained from the results above.
Date posted: May 23, 2019. Answers (1)
- Use the bond energies given below to calculate the heat of reaction for;(Solved)
Use the bond energies given below to calculate the heat of reaction for;

Date posted: May 23, 2019. Answers (1)
- Study the diagram below then use it to answer the questions that follow.
a) Draw the wooden splint at the end of the experiment. If it...(Solved)
Study the diagram below then use it to answer the questions that follow.

a) Draw the wooden splint at the end of the experiment. If it was slipped then removed.
b) Explain the appearance of the wooden splint in (a) above.
Date posted: May 23, 2019. Answers (1)
- Which is the chemical test to differentiate between alkenes and alkynes?(Solved)
Which is the chemical test to differentiate between alkenes and alkynes?
Date posted: May 22, 2019. Answers (1)
- Use the table of logarithms to evaluate. (Solved)
Use the table of logarithms to evaluate.

Date posted: May 22, 2019. Answers (1)
- The diagram below shows acidic and basic oxides fit in a general family of oxides.
a) State the name given to the type of oxides that...(Solved)
The diagram below shows acidic and basic oxides fit in a general family of oxides.

a) State the name given to the type of oxides that would be placed in the shaded region.
b) Name two oxides that could be placed on the shaded region.
Date posted: May 22, 2019. Answers (1)
- Substances X and Y consists of molecules X2 and Y2 respectively. When the two
elements react, they form a molecule of XY. The X-X bonds are...(Solved)
Substances X and Y consists of molecules X2 and Y2 respectively. When the two
elements react, they form a molecule of XY. The X-X bonds are as strong as Y-Y bonds.
But the X-Y bond is stronger than both X-X and Y-Y bonds.

a) Is the above reaction exothermic or endothermic? Give a reason for your answer
b) Draw an energy level diagram for the reaction in (a) above.
Date posted: May 22, 2019. Answers (1)