Because intermolecular forces in gases are weaker than in solids.
sharon kalunda answered the question on May 30, 2019 at 05:44
- The figure shows a U-tube connected to gas supply containing liquids L1 and L2 of densities 1.8gcm-3 and 0.8gcm-3 respectively in equilibrium.
Given that h1 =...(Solved)
The figure shows a U-tube connected to gas supply containing liquids L1 and L2 of densities 1.8gcm-3 and 0.8gcm-3 respectively in equilibrium.

Given that h1 = 8cm, h2 = 10cm and atmospheric pressure is 1.02 x 105Pa. Determine the gas pressure.
Date posted: May 30, 2019. Answers (1)
- Give a reason why mercury is preferred for use in a thermometer.(Solved)
Give a reason why mercury is preferred for use in a thermometer.
Date posted: May 30, 2019. Answers (1)
- The figure below shows a velocity-time graph for a body.
Describe the motion of the body between A and B.(Solved)
The figure below shows a velocity-time graph for a body.

Describe the motion of the body between A and B.
Date posted: May 30, 2019. Answers (1)
- The figure below shows a glass tube dipped in water inside a trough. The cross sectional area of the tube is 2cm2.
Determine the adhesive...(Solved)
The figure below shows a glass tube dipped in water inside a trough. The cross sectional area of the tube is 2cm2.

Determine the adhesive force between water and glass. (Take density of water = 1000kgm-3, acceleration due to gravity =
10ms-2).
Date posted: May 30, 2019. Answers (1)
- The figure below shows a burette containing a liquid up to the level marked on the figure. 20 drops of the liquid are now run...(Solved)
The figure below shows a burette containing a liquid up to the level marked on the figure. 20 drops of the liquid are now run out of the burette. If each drop has a volume of 1.1ml. Mark on the figure the new level of the liquid in the burette.

Date posted: May 30, 2019. Answers (1)
- A generator produces 0.1MW which is transmitted through a cable of resistance 10 ohms. If the potential difference produced is 5KV, calculate current transmitted.(Solved)
A generator produces 0.1MW which is transmitted through a cable of resistance 10 ohms. If the potential
difference produced is 5KV, calculate current transmitted.
Date posted: May 29, 2019. Answers (1)
- Define the term ‘kilowatt hour’(Solved)
Define the term ‘kilowatt hour’
Date posted: May 29, 2019. Answers (1)
- The figure below shows a compound microscope where by O is object, I is the first image and I2 is the final image. A is
objective...(Solved)
The figure below shows a compound microscope where by O is object, I is the first image and I2 is the final image. A is
objective lens while E is the eyepiece.

Find the total magnification.
Date posted: May 29, 2019. Answers (1)
- convex lens forms an image four times the size of the object on a screen. If the distance between the object and the
screen is...(Solved)
A convex lens forms an image four times the size of the object on a screen. If the distance between the object and the
screen is 10cm.
Determine :
i) the image distance.
ii) focal length of the lens.
Date posted: May 29, 2019. Answers (1)
- The figure below show an X-ray tube.
b)i) Briefly explain how X-rays are produced.
ii) What adjustment should be made on the tube to produce hard...(Solved)
The figure below show an X-ray tube.
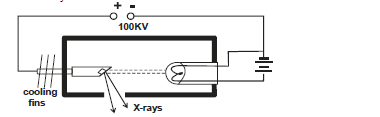
a)i) Briefly explain how X-rays are produced.
ii) What adjustment should be made on the tube to produce hard X-rays if initially it was producing soft X-rays.
b) What are the functions of the following on the cathode ray oscilloscope ?
i) X-plates.
ii) Grid.
Date posted: May 29, 2019. Answers (1)
- A radio station is transmitting at a frequency of 15MHz. Calculate the wavelength of the transmission.(Solved)
A radio station is transmitting at a frequency of 15MHz. Calculate the wavelength of the transmission.
Date posted: May 29, 2019. Answers (1)
- Arrange the electromagnetic wave below in ascending order of wavelength. visible light, infrared, ultraviolet, radio
waves, gamma rays, x-rays.(Solved)
Arrange the electromagnetic wave below in ascending order of wavelength. visible light, infrared, ultraviolet, radio
waves, gamma rays, x-rays.
Date posted: May 29, 2019. Answers (1)
- In a transformer, a voltage of 240V is to be stepped down to 24V. The primary current is found to be 1.5A while thesecondary current...(Solved)
In a transformer, a voltage of 240V is to be stepped down to 24V. The primary current is found to be 1.5A while the
secondary current is 14A. Calculate :
i) Power input
ii) Power output
iii) Power wasted
iv) Efficiency of the transformer
Date posted: May 29, 2019. Answers (1)
- The figure below shows one method of a.c rectification using four diodes, A, B, C, D.
Explain how the a.c rectification is achieved in the above...(Solved)
The figure below shows one method of a.c rectification using four diodes, A, B, C, D.

Explain how the a.c rectification is achieved in the above circuit.
Date posted: May 29, 2019. Answers (1)
- What is meant by the term “A.C rectification”.(Solved)
What is meant by the term “A.C rectification”.
Date posted: May 29, 2019. Answers (1)
- In the nuclear equation below, find a and b(Solved)
In the nuclear equation below, find a and b

Date posted: May 29, 2019. Answers (1)
- The figure below shows a Geiger Muller (GM) tube.
i) Name the part labelled A and B
ii) State one reason why halogen gas is used...(Solved)
The figure below shows a Geiger Muller (GM) tube.
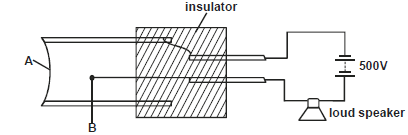
i) Name the part labelled A and B
ii) State one reason why halogen gas is used in the GM tube.
Date posted: May 29, 2019. Answers (1)
- In an experiment to determine the half life of thoron 220, the results were plotted as shown.
From the graph, determine the half life of thoron...(Solved)
In an experiment to determine the half life of thoron 220, the results were plotted as shown.

From the graph, determine the half life of thoron 220.
Date posted: May 29, 2019. Answers (1)
- A certain radioactive material has half life of 20 minutes. Calculate the fraction of the original mass that would be
remaining after two hours.(Solved)
A certain radioactive material has half life of 20 minutes. Calculate the fraction of the original mass that would be
remaining after two hours.
Date posted: May 29, 2019. Answers (1)
- The figure below shows the deflection of radiation from a radioactive substance by an electric field.
Identify the radiations P, Q and R(Solved)
The figure below shows the deflection of radiation from a radioactive substance by an electric field.
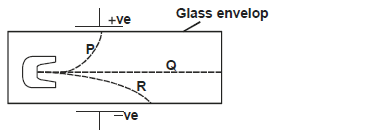
Identify the radiations P, Q and R
Date posted: May 29, 2019. Answers (1)