Ice requires latent to melt it, but water at 0°C does not require the latent heat
sharon kalunda answered the question on May 30, 2019 at 09:35
- When graphite particles are suspended in water and observed through a microscope, they are seen to move in a random
motion. Explain.(Solved)
When graphite particles are suspended in water and observed through a microscope, they are seen to move in a random
motion. Explain.
Date posted: May 30, 2019. Answers (1)
- A uniform rod of length 4m and mass of 4kg is pivoted at 3.6m mark. The rod is held horizontal with a vertical rope at...(Solved)
A uniform rod of length 4m and mass of 4kg is pivoted at 3.6m mark. The rod is held horizontal with a vertical rope at the 4m
mark, as shown in the figure 3.0 below.
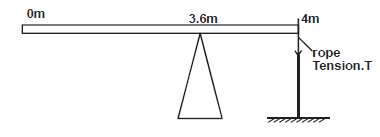
Calculate the tension, T in the rope (Take g = 10N/kg)
Date posted: May 30, 2019. Answers (1)
- The diagram below shows a rectangular wire with loose thread tied in it and dipped in a soap solution to form a film.
Draw a diagram...(Solved)
The diagram below shows a rectangular wire with loose thread tied in it and dipped in a soap solution to form a film.
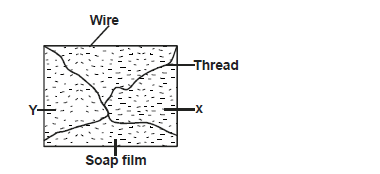
Draw a diagram showing what will be observed when the film is broken at points X and Y.
Date posted: May 30, 2019. Answers (1)
- A car of mass 800kg moves on a circular track of radius 20m. The force of friction between the tyres and the tarmac is
4800N. Determine...(Solved)
A car of mass 800kg moves on a circular track of radius 20m. The force of friction between the tyres and the tarmac is
4800N. Determine the maximum speed at which the car can be driven on the track without skidding.
Date posted: May 30, 2019. Answers (1)
- A Spring extends by 0.7cm when a mass of 420g is hang on it on earth. By what length would the spring extend if the...(Solved)
A Spring extends by 0.7cm when a mass of 420g is hang on it on earth. By what length would the spring extend if the same
set up was taken to the moon where the gravitational intensity is one-sixth of that one earth.Take gravitational field intensity on surface of Earth g, 10N/kg.
Date posted: May 30, 2019. Answers (1)
- A density bottle was used to measure the density of liquid L and the following were the measurements taken
- Mass of empty bottle = 26g
-...(Solved)
A density bottle was used to measure the density of liquid L and the following were the measurements taken
- Mass of empty bottle = 26g
- Mass of bottle filled with alcohol (of density 800kg/m³) = 66g
- Mass of bottle filled with liquid L - 86g
Find the density of liquid L.
Date posted: May 30, 2019. Answers (1)
- The graph shows the activity versus time for a sample of radioactive material.
Use the graph to determine :
i) the half life of the sample
ii)...(Solved)
The graph shows the activity versus time for a sample of radioactive material.
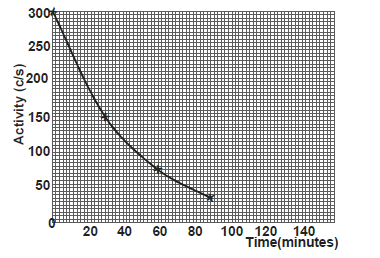
Use the graph to determine :
i) the half life of the sample
ii) the number of half lifes needed for the activity to reduce from 300C/S to 37.5C/S
Date posted: May 30, 2019. Answers (1)
- State what is observed on the screen of a CRO when :
i) low voltage alternating current is connected to the time base and the y-gain...(Solved)
State what is observed on the screen of a CRO when :
i) low voltage alternating current is connected to the time base and the y-gain switched off.
ii) a high voltage ac is connected to the y-gain and the time base is switched off.
Date posted: May 30, 2019. Answers (1)
- An object forms a virtual image three times the size of the object. If the object is placed 10cm from the lens.
Determine :
i) the image...(Solved)
An object forms a virtual image three times the size of the object. If the object is placed 10cm from the lens.
Determine :
i) the image distance from lens.
ii) the focal length of the lens
Date posted: May 30, 2019. Answers (1)
- The figure below shows a path of a ray of light through a glass prism. The refractive index of glass is 1.52 and the rays
makes...(Solved)
The figure below shows a path of a ray of light through a glass prism. The refractive index of glass is 1.52 and the rays
makes an angle of 40o with the prism.
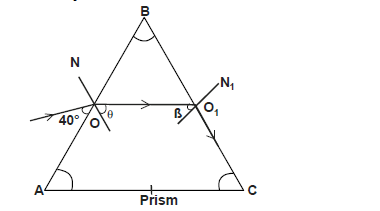
ON and O1N1 are normals to the prism at the faces AB and BC respectively. If the speed of light in air is 3 x 108m/s. Determine :
i) the speed of light in glass
ii) the angles marked
I. angle theta
II. angle beta
Date posted: May 30, 2019. Answers (1)
- Name a device that can be used to improve on the nature of the output current.(Solved)
Name a device that can be used to improve on the nature of the output current.
Date posted: May 30, 2019. Answers (1)
- The figure below shows four pieces of a device used in full wave rectification.
i) Name the devices B1, B2, B3 and B4
ii) Complete the...(Solved)
The figure below shows four pieces of a device used in full wave rectification.
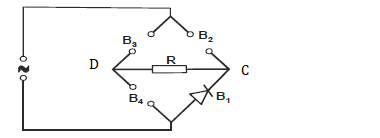
i) Name the devices B1, B2, B3 and B4
ii) Complete the diagram by showing the correct directions of B2, B3 and B4
iii) Use an arrow to show the direction of current flow through R.
iv) Sketch the output current as would be observed on the screen of a CRO fixed between C and D.
Date posted: May 30, 2019. Answers (1)
- In an X-ray tube, the electrons are accelerated by a p.d of 24000V. Assuming that 2% of energy produced is converted
to X-rays. Determine the :
i)...(Solved)
In an X-ray tube, the electrons are accelerated by a p.d of 24000V. Assuming that 2% of energy produced is converted
to X-rays. Determine the :
i) energy of the X-rays produced.
ii) frequency of X-rays produced (take planks constant h = 6.6 x 10-34Js and charge on an electron e= 1.6 x 10-19C)
Date posted: May 30, 2019. Answers (1)
- The figure below shows the circuits close to each other.
When the switch is closed, the galvanometer shows a reading and then returns to zero.
i) Explain...(Solved)
The figure below shows the circuits close to each other.
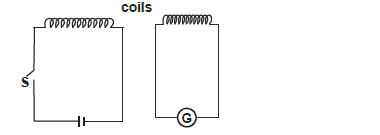
When the switch is closed, the galvanometer shows a reading and then returns to zero.
i) Explain the observation
ii) Give one adjustment that can be done to the arrangement so that
I. the galvanometer gives a bigger deflection in the same direction.
II. the galvanometer deflects in opposite direction when the switch is closed.
Date posted: May 30, 2019. Answers (1)
- The figure below shows an electrical circuit with three capacitors A, B and C.
Determine :
i) the current flowing in the system.
ii) the total...(Solved)
The figure below shows an electrical circuit with three capacitors A, B and C.
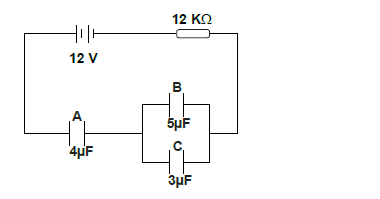
Determine :
i) the current flowing in the system.
ii) the total capacitance of the capacitors.
iii) the total charge stored in the capacitors.
iv) The time needed to fully charge the capacitors.
Date posted: May 30, 2019. Answers (1)
- The figure below shows two parallel plate capacitors connected to a battery. Initially the switch S is open.
The switch is now closed and left for...(Solved)
The figure below shows two parallel plate capacitors connected to a battery. Initially the switch S is open.
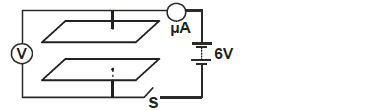
The switch is now closed and left for a few seconds.
i) In the space below sketch a graph of current reading with time from time the switch is closed.
ii) Determine the reading of V after a long time.
iii) How does the capacitance of the plates change when the plates are moved further apart.
Date posted: May 30, 2019. Answers (1)
- The figure below shows an object and its image on a concave mirror. It is drawn to a scale of 1 : 10
Using a ray...(Solved)
The figure below shows an object and its image on a concave mirror. It is drawn to a scale of 1 : 10
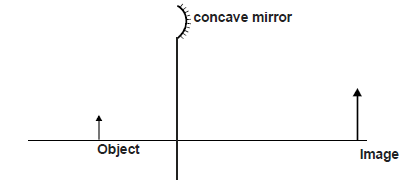
Using a ray from the object, determine
a) the position of the principal focus.
b) the focal length of the mirror
Date posted: May 30, 2019. Answers (1)
- Uranium U 234 decays to Polonium P 218 by emitting alpha particles. Determine the number of alpha particles emitted.(Solved)
Uranium U 234 decays to Polonium P 218 by emitting alpha particles. Determine the number of alpha particles emitted.
Date posted: May 30, 2019. Answers (1)
- Name one electromagnetic wave whose energy is higher than that of visible light.(Solved)
Name one electromagnetic wave whose energy is higher than that of visible light.
Date posted: May 30, 2019. Answers (1)
- The table below shows some electrical appliances to be used in a house. The electrical rating for each appliance is shown.
a) Determine the resistance of...(Solved)
The table below shows some electrical appliances to be used in a house. The electrical rating for each appliance is shown.
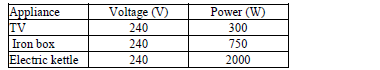
a) Determine the resistance of the coil of the TV set.
b) Determine the appropriate current of each fuse used in the house.
Date posted: May 30, 2019. Answers (1)