- Carbon dioxide is used to make fizzy drinks. It is stored in high pressure in cast iron cylinder. The figure below represents the
particles in a...(Solved)
Carbon dioxide is used to make fizzy drinks. It is stored in high pressure in cast iron cylinder. The figure below represents the
particles in a cylinder of carbon dioxide.
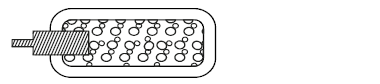
Describe how particles of carbon dioxide exert pressure.
Date posted: May 30, 2019. Answers (1)
- The figure below shows a funnel dipped into a liquid soap solution.
Explain what happens to the soap bubble when the soap is removed.(Solved)
The figure below shows a funnel dipped into a liquid soap solution.
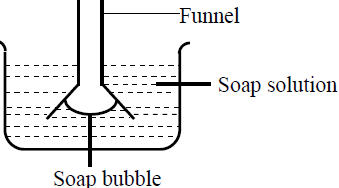
Explain what happens to the soap bubble when the soap is removed.
Date posted: May 30, 2019. Answers (1)
- A solid displaces 8.5cm³ of liquid when floating on a certain liquid and 11.5cm³ when fully submerged with liquid. The
density of solid in 0.8g/cm³, determine:-
i)...(Solved)
A solid displaces 8.5cm³ of liquid when floating on a certain liquid and 11.5cm³ when fully submerged with liquid. The
density of solid in 0.8g/cm³, determine:-
i) Upthrust on the solid when floating.
ii) Density of the liquid.
Date posted: May 30, 2019. Answers (1)
- The figure below shows an empty beaker placed on the top of a pan calibrated in grammes. 50ml of alcohol of density 0.8g/cm3 was added...(Solved)
The figure below shows an empty beaker placed on the top of a pan calibrated in grammes. 50ml of alcohol of density 0.8g/cm3 was added to the beaker.
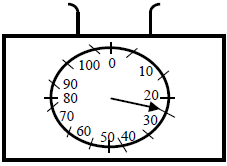
Show on the figure the new pointer position.
Date posted: May 30, 2019. Answers (1)
- The figure shows a piece of cork held with a light thread attached to the bottom of a beaker. The beaker is filled with
water.
i) Indicate...(Solved)
The figure shows a piece of cork held with a light thread attached to the bottom of a beaker. The beaker is filled with
water.
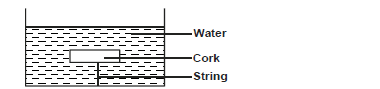
i) Indicate and label on the diagram the forces acting on the cork.
ii) Write an expression showing the relationship between the forces.
Date posted: May 30, 2019. Answers (1)
- On the set of axes below show how the volume of an ideal gas varies with pressure.(Solved)
On the set of axes below show how the volume of an ideal gas varies with pressure.
Date posted: May 30, 2019. Answers (1)
- The figure below shows the use of pulleys in holding a cable taut.
a) What is the purpose of pulley X?
b) How do the pulleys...(Solved)
The figure below shows the use of pulleys in holding a cable taut.
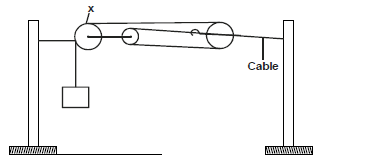
a) What is the purpose of pulley X?
b) How do the pulleys used serve the purpose of the arrangement?
Date posted: May 30, 2019. Answers (1)
- A girl blew air along the horizontal plane below the paper as shown in figure below.
State and explain what would be observed.(Solved)
A girl blew air along the horizontal plane below the paper as shown in figure below.
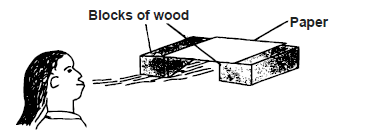
State and explain what would be observed.
Date posted: May 30, 2019. Answers (1)
- The figure below shows a ball being whirled in a vertical plane.
Sketch on the figure the path followed by the ball if the string cuts...(Solved)
The figure below shows a ball being whirled in a vertical plane.
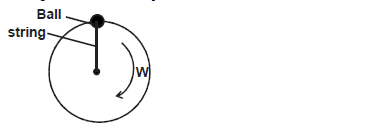
Sketch on the figure the path followed by the ball if the string cuts when the ball is in the position shown in the figure.
Date posted: May 30, 2019. Answers (1)
- A motor car is uniformly retarded and brought to rest from a speed of 108 km/h in 15 sec. Find its acceleration.(Solved)
A motor car is uniformly retarded and brought to rest from a speed of 108 km/h in 15 sec. Find its acceleration.
Date posted: May 30, 2019. Answers (1)
- The figure shows an instrument used to measure atmospheric pressure.
a) Name the instrument.
b) Name the liquid marked L.(Solved)
The figure shows an instrument used to measure atmospheric pressure.
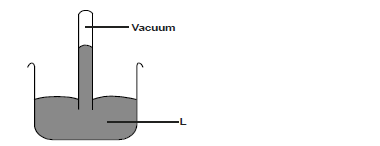
a) Name the instrument.
b) Name the liquid marked L.
Date posted: May 30, 2019. Answers (1)
- A man has 2m³ of concrete delivered to his home and he needed to carry it down in a wheelbarrow. If each barrow load weighs...(Solved)
A man has 2m³ of concrete delivered to his home and he needed to carry it down in a wheelbarrow. If each barrow load weighs 2500N, how many trips will he have to make?
(Density of concrete = 3000kgm-3).
Date posted: May 30, 2019. Answers (1)
- The figure below shows a section of a vernier calliper scale.
State the reading of the diameter being measured.(Solved)
The figure below shows a section of a vernier calliper scale.
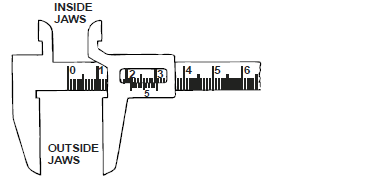
State the reading of the diameter being measured.
Date posted: May 30, 2019. Answers (1)
- A radioactive element X of half-life of 28 days decay to element Y. A sample of X of mass 16g is kept in a container.
Assuming...(Solved)
A radioactive element X of half-life of 28 days decay to element Y. A sample of X of mass 16g is kept in a container.
Assuming Y is stable, determine the mass of Y that will be found in the containers after 112 days.
Date posted: May 30, 2019. Answers (1)
- The figure below shows a ray of white light dispersed in a triangular prism. The speed of violet light in the prism in 1.88 ×...(Solved)
The figure below shows a ray of white light dispersed in a triangular prism. The speed of violet light in the prism in 1.88 × 108m/s.
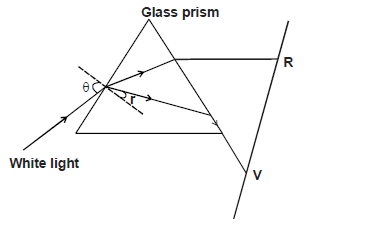
a) Explain how glass disperses white light into red and violet bands.
b) Determine the refractive index of the prism material for light (take speed of light in vacuum = 3×108m/s
c) Show on the figure the critical angle C for violet light and determine its value.
d) Given that r =21.5° determine the angle
e) On the same figure, sketch the part of red light after white light strikes the prism if the prism was replaced by another of
similar shape but lower refractive index. (Use a dotted line for the answer)
Date posted: May 30, 2019. Answers (1)
- Determine the cost of using an electric iron rated 1500W, for a total of 30 hours given that the cost of electricity per kwhis kshs...(Solved)
Determine the cost of using an electric iron rated 1500W, for a total of 30 hours given that the cost of electricity per kwh is kshs 8.
Date posted: May 30, 2019. Answers (1)
- A house has four lamps rated (60W, 240V), a cooker rated (5kw, 240V), and an iron box rated (1kW, 240V). All are used 3.5 hours...(Solved)
A house has four lamps rated (60W, 240V), a cooker rated (5kw, 240V), and an iron box rated (1kW, 240V). All are used 3.5 hours a day. Calculate the monthly cost for electricity for the owner at the rate of 13.5 cents/ KWhr.
Date posted: May 30, 2019. Answers (1)
- Figure below shows a simple barometer.
a) What is the region A?
b) What keeps the mercury in the tube?
c) What is the value of the...(Solved)
Figure below shows a simple barometer.
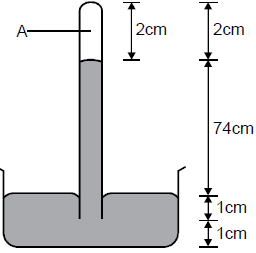
a) What is the region A?
b) What keeps the mercury in the tube?
c) What is the value of the atmospheric pressure being shown by the barometer?
d) What would happen to the reading if the barometer were taken up a high mountain.
e) Give a reason for (d) above.
Date posted: May 30, 2019. Answers (1)
- The diagram below shows a section of a micrometer screw gauge.
The thimble of the micrometer screw gauge is rotated through two and half revolutions in...(Solved)
The diagram below shows a section of a micrometer screw gauge.
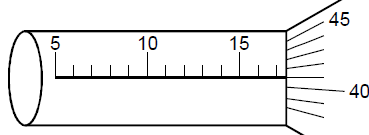
The thimble of the micrometer screw gauge is rotated through two and half revolutions in the clockwise direction in order to measure the diameter of a marble. State the diameter of the marble if the instrument had a negative error of
0.03mm.
Date posted: May 30, 2019. Answers (1)
- The primary coil of a transformer has 1200 turns and the secondary coil has 60 turns. The transformer is connected to a 240 V a.c source. (i) The output...(Solved)
The primary coil of a transformer has 1200 turns and the secondary coil has 60 turns. The transformer is connected to a 240V a.c source.
i) The output voltage.
ii) The output current when the primary coil has a current of 0.5A (Assume there is no energy losses)
iii) One of the primary ways in which power is lost in transformers is through eddy currents. State how eddy currents can be
minimized.
Date posted: May 30, 2019. Answers (1)