-
The expression below is an equation for a radioactive element P. Element Q and R are the daughter nuclides. P, Q and R are not...
(Solved)
The expression below is an equation for a radioactive element P. Element Q and R are the daughter nuclides. P, Q and R are not the actual symbols of any of the elements.
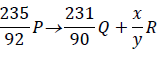
Identify the element R and state two of its characteristics.
Date posted:
May 31, 2019
.
Answers (1)
-
The mass of the fabric of a large balloon is 100kg. The balloon is inflated with 200cm3 of helium. The balloon is attached to a...
(Solved)
The mass of the fabric of a large balloon is 100kg. The balloon is inflated with 200cm3 of helium. The balloon is attached to a cable fixed to the ground as shown.
(Density of air and helium are 1.25kg/m3 and 0.2kg/m3 respectively)
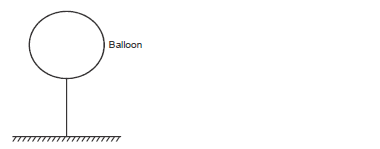
i) Indicate all the forces acting on the system.
ii) If the system is at equilibrium, write an equation relating the three forces in (i) above.
iii) Calculate the upthrust on the balloon.
Date posted:
May 31, 2019
.
Answers (1)
-
A motorcycle is travelling at a constant speed of 72km/h around a circular track of radius 150m.
i) Determine its centripetal acceleration.
ii) How long does...
(Solved)
A motorcycle is travelling at a constant speed of 72km/h around a circular track of radius 150m.
i) Determine its centripetal acceleration.
ii) How long does the cyclist take to complete one full cycle of the track?
Date posted:
May 31, 2019
.
Answers (1)
-
A column of glycerine 8.20m high, a column of sea water 10.08m high, column of mercury 0.76m high and a column of fresh water 10.34m...
(Solved)
A column of glycerine 8.20m high, a column of sea water 10.08m high, column of mercury 0.76m high and a column of fresh water 10.34m high exert the same pressure at the bottom of the container.Given that the pressure exerted by a fluid is given by P = h g, arrange these liquid in decreasing order of their densities.
Date posted:
May 31, 2019
.
Answers (1)
-
5 images are formed when two mirrors are inclined at an angle between them. Determine the angle of inclination.
(Solved)
5 images are formed when two mirrors are inclined at an angle between them. Determine the angle of inclination.
Date posted:
May 31, 2019
.
Answers (1)
-
A stone of mass 450g is rotated in a vertical circle at 3 revolutions per second. If the string has a length of 1.5m,
determine:
(i) the...
(Solved)
A stone of mass 450g is rotated in a vertical circle at 3 revolutions per second. If the string has a length of 1.5m,
determine:
(i) the linear velocity
(ii) The tension of the string at positions A and B.
Date posted:
May 31, 2019
.
Answers (1)
-
Two equal masses travel towards each other on a frictionless air track at speeds of 60cm/s and 40cm /s. They stick together on impact.
What is...
(Solved)
Two equal masses travel towards each other on a frictionless air track at speeds of 60cm/s and 40cm /s. They stick together on impact.

What is the velocity of the masses after impact?
Date posted:
May 31, 2019
.
Answers (1)
-
The graph below shows the variation of force with distance for a body being towed.
Calculate the total work done on the body.
(Solved)
The graph below shows the variation of force with distance for a body being towed.
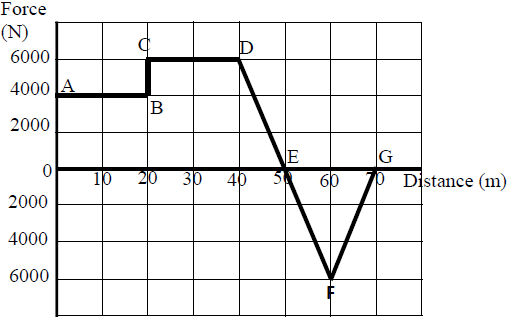
Calculate the total work done on the body.
Date posted:
May 31, 2019
.
Answers (1)
-
Two gear wheel have a 80 teeth (driven) and 20 teeth (driving) and lock with each other. They are fastened on axles of
equal diameters such...
(Solved)
Two gear wheel have a 80 teeth (driven) and 20 teeth (driving) and lock with each other. They are fastened on axles of
equal diameters such that a weight of 150N attached to a string round one axle will just raise 450N on the other axle.
Calculate
(i) M.A
(ii) V.R
(iii) Efficiency of the machine.
Date posted:
May 31, 2019
.
Answers (1)
-
The figure below shows a car braking system. The brake fluid is an oily liquid.
The brake drum rotates with the wheel of the car.
(i) Explain...
(Solved)
The figure below shows a car braking system. The brake fluid is an oily liquid.
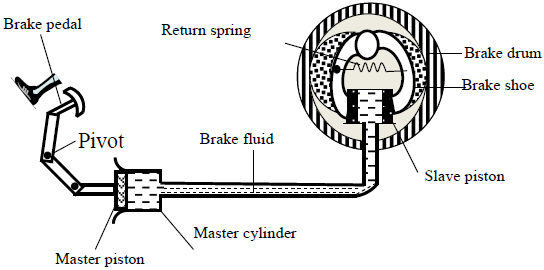
The brake drum rotates with the wheel of the car.
(i) Explain how pushing the brake pedal makes the brake rub against the drum.
(ii) The cross-sectional area of the master piston is 2.0cm2. A force of 140N is applied to the master piston.
(I) Calculate the pressure created in the brake fluid by the master piston.
(II) The cross-sectional area of each slave piston is 2.8cm2. Calculate the force exerted on each slave piston by the brake fluid.
(III) The force exerted on the master piston is greater than the force applied by the foot on the brake pedal. Using the principle of moments, explain this
Date posted:
May 31, 2019
.
Answers (1)
-
The figure below shows a bottle opener.
A force of 30N is applied at a distance of 11cm from the pivot P. The force F on...
(Solved)
The figure below shows a bottle opener.
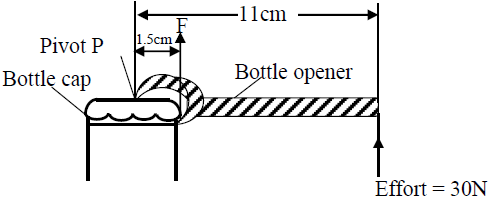
A force of 30N is applied at a distance of 11cm from the pivot P. The force F on the bottle cap of 1.5cm from the pivot P. Calculate the force F on the edge of the cap.
Date posted:
May 31, 2019
.
Answers (1)
-
A heater is rated 3kW, 240V. The fuses available are marked 10A, 13A and 20A. Which fuse is most suitable?
(Solved)
A heater is rated 3kW, 240V. The fuses available are marked 10A, 13A and 20A. Which fuse is most suitable?
Date posted:
May 30, 2019
.
Answers (1)
-
How many electric iron boxes rated 1000W could be safely connected to a 240V mains circuit fitted with a 13A fuse?
(Solved)
How many electric iron boxes rated 1000W could be safely connected to a 240V mains circuit fitted with a 13A fuse?
Date posted:
May 30, 2019
.
Answers (1)
-
A ferromagnetic material is being magnetised by single stroking method. On the axes provided, sketch a graph to show how
the strength of the magnet being...
(Solved)
A ferromagnetic material is being magnetised by single stroking method. On the axes provided, sketch a graph to show how
the strength of the magnet being created varies with number of strokes.
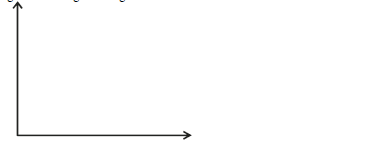
Date posted:
May 30, 2019
.
Answers (1)
-
A metal cube weighs 1.0N in air and 0.8N when totally immersed in water.
Calculate
(i) Volume of water it displaces.
(ii) the density of the cube
(Solved)
A metal cube weighs 1.0N in air and 0.8N when totally immersed in water.
Calculate
(i) Volume of water it displaces.
(ii) the density of the cube
Date posted:
May 30, 2019
.
Answers (1)
-
A boy on a bicycle accelerated uniformly at 1m/s2 for 10 seconds from an initial velocity of 4m/s. Calculate the distance travelled in this time.
(Solved)
A boy on a bicycle accelerated uniformly at 1m/s2 for 10 seconds from an initial velocity of 4m/s. Calculate the distance travelled in this time.
Date posted:
May 30, 2019
.
Answers (1)
-
The figure below shows a funnel dipped into a liquid soap solution.
Explain what happens to the soap bubble when the soap is removed.
(Solved)
The figure below shows a funnel dipped into a liquid soap solution.
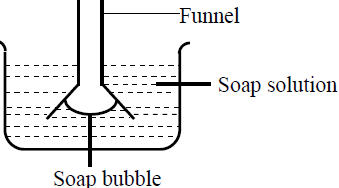
Explain what happens to the soap bubble when the soap is removed.
Date posted:
May 30, 2019
.
Answers (1)
-
A solid displaces 8.5cm³ of liquid when floating on a certain liquid and 11.5cm³ when fully submerged with liquid. The
density of solid in 0.8g/cm³, determine:-
i)...
(Solved)
A solid displaces 8.5cm³ of liquid when floating on a certain liquid and 11.5cm³ when fully submerged with liquid. The
density of solid in 0.8g/cm³, determine:-
i) Upthrust on the solid when floating.
ii) Density of the liquid.
Date posted:
May 30, 2019
.
Answers (1)
-
The figure below shows an empty beaker placed on the top of a pan calibrated in grammes. 50ml of alcohol of density 0.8g/cm3 was added...
(Solved)
The figure below shows an empty beaker placed on the top of a pan calibrated in grammes. 50ml of alcohol of density 0.8g/cm3 was added to the beaker.
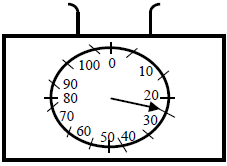
Show on the figure the new pointer position.
Date posted:
May 30, 2019
.
Answers (1)
-
A motor car is uniformly retarded and brought to rest from a speed of 108 km/h in 15 sec. Find its acceleration.
(Solved)
A motor car is uniformly retarded and brought to rest from a speed of 108 km/h in 15 sec. Find its acceleration.
Date posted:
May 30, 2019
.
Answers (1)