-
A car of mass 800g is initially moving at 25m/s. Calculate the force needed to bring the car to rest over a distance of 20m.
(Solved)
A car of mass 800g is initially moving at 25m/s. Calculate the force needed to bring the car to rest over a distance of 20m.
Date posted:
May 31, 2019
.
Answers (1)
-
In an experiment to determine the thickness of an oil molecule, an oil drop of volume 3.60 x 10-6 m3 was observed to form a...
(Solved)
In an experiment to determine the thickness of an oil molecule, an oil drop of volume 3.60 x 10-6 m3 was observed to form a circular patch of diameter 0.016m on the surface of water covered with lycopodium powder
i). Explain why the oil drop forms a circular patch.
ii) Determine the thickness of the oil molecule
Date posted:
May 31, 2019
.
Answers (1)
-
The figure below shows a uniform plank AB of length 10m weighing 500N. Two masses measuring 25kg and 60kg are loaded on its ends.
Determine the...
(Solved)
The figure below shows a uniform plank AB of length 10m weighing 500N. Two masses measuring 25kg and 60kg are loaded on its ends.

Determine the distance from point A where a support should be placed for the plank to balance horizontally.
Date posted:
May 31, 2019
.
Answers (1)
-
A small drop of oil has a volume of 5 × 10-8m³. When it is put on the surface of some clean water, it forms...
(Solved)
A small drop of oil has a volume of 5 × 10-8m³. When it is put on the surface of some clean water, it forms a circular film of 0.1m² in area; what is the size the a molecule oil.
Date posted:
May 31, 2019
.
Answers (1)
-
The distance between the ice point and steam point on a liquid in glass thermometer is 30cm. what temperature is recorded when the mercury thread...
(Solved)
The distance between the ice point and steam point on a liquid in glass thermometer is 30cm. what temperature is recorded when the mercury thread is 12cm above the ice point?
Date posted:
May 31, 2019
.
Answers (1)
-
A single spring stretches by 2.0 cm when supporting a load of 50N. If in the system below the springs are identical and have negligible...
(Solved)
A single spring stretches by 2.0 cm when supporting a load of 50N. If in the system below the springs are identical and have negligible weight
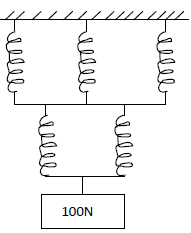
Find:
a) The total extension of the system.
b) The total spring constant.
Date posted:
May 31, 2019
.
Answers (1)
-
The diagram below shows a micrometer screw gauge used by a student to measure the thickness of a wire. If it has a zero error...
(Solved)
The diagram below shows a micrometer screw gauge used by a student to measure the thickness of a wire. If it has a zero error of 0.06mm, what is the actual thickness of the wire?
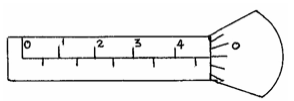
Date posted:
May 31, 2019
.
Answers (1)
-
A transformer is used to provide a potential difference of 100KV to an X-ray tube from 250V a.c mains supply. A current of 100mA flows...
(Solved)
A transformer is used to provide a potential difference of 100KV to an X-ray tube from 250V a.c mains supply. A current of 100mA flows in ht X-ray tube and the transformer is 100% efficient. Calculate:-
(i) The ratio of the number of turns of the secondary coil to the number of turns in the primary coil.
(ii) The current in the primary coil.
(iii) State giving reasons which of the coils of the transformer is thinner.
Date posted:
May 31, 2019
.
Answers (1)
-
In an experiment to determine the refractive index of a liquid, the liquid was poured into a measuring cylinder, a pin was
placed at the bottom...
(Solved)
In an experiment to determine the refractive index of a liquid, the liquid was poured into a measuring cylinder, a pin was
placed at the bottom of the cylinder and another pin was used to locate the apparent position of the first pin. The values
of real and apparent depth were used to plot a graph in the figure below.
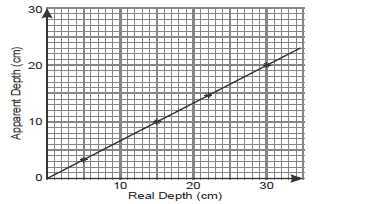
i) From the graph determine the refractive index of the liquid.
ii) Given that the velocity of light in vacuum is 3.0 x 108 m/s what would be the velocity of light in the liquid
above.
Date posted:
May 31, 2019
.
Answers (1)
-
The coil of an electric motor is usually wound on a soft iron armature. State two purposes of this armature.
(Solved)
The coil of an electric motor is usually wound on a soft iron armature. State two purposes of this armature.
Date posted:
May 31, 2019
.
Answers (1)
-
A 200g mass of copper was heated to 100°C and then transferred to a lagged copper calorimeter of mass 5g containing 125g of water at...
(Solved)
A 200g mass of copper was heated to 100°C and then transferred to a lagged copper calorimeter of mass 5g containing 125g of water at 30°C. Calculate the final temperature of water. (Specific heat capacity of copper = 400J/KgK, water =4200J/KgK).
Date posted:
May 31, 2019
.
Answers (1)
-
The expression below is an equation for a radioactive element P. Element Q and R are the daughter nuclides. P, Q and R are not...
(Solved)
The expression below is an equation for a radioactive element P. Element Q and R are the daughter nuclides. P, Q and R are not the actual symbols of any of the elements.
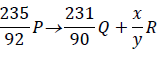
Identify the element R and state two of its characteristics.
Date posted:
May 31, 2019
.
Answers (1)
-
The mass of the fabric of a large balloon is 100kg. The balloon is inflated with 200cm3 of helium. The balloon is attached to a...
(Solved)
The mass of the fabric of a large balloon is 100kg. The balloon is inflated with 200cm3 of helium. The balloon is attached to a cable fixed to the ground as shown.
(Density of air and helium are 1.25kg/m3 and 0.2kg/m3 respectively)
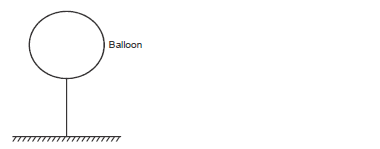
i) Indicate all the forces acting on the system.
ii) If the system is at equilibrium, write an equation relating the three forces in (i) above.
iii) Calculate the upthrust on the balloon.
Date posted:
May 31, 2019
.
Answers (1)
-
A motorcycle is travelling at a constant speed of 72km/h around a circular track of radius 150m.
i) Determine its centripetal acceleration.
ii) How long does...
(Solved)
A motorcycle is travelling at a constant speed of 72km/h around a circular track of radius 150m.
i) Determine its centripetal acceleration.
ii) How long does the cyclist take to complete one full cycle of the track?
Date posted:
May 31, 2019
.
Answers (1)
-
A column of glycerine 8.20m high, a column of sea water 10.08m high, column of mercury 0.76m high and a column of fresh water 10.34m...
(Solved)
A column of glycerine 8.20m high, a column of sea water 10.08m high, column of mercury 0.76m high and a column of fresh water 10.34m high exert the same pressure at the bottom of the container.Given that the pressure exerted by a fluid is given by P = h g, arrange these liquid in decreasing order of their densities.
Date posted:
May 31, 2019
.
Answers (1)
-
5 images are formed when two mirrors are inclined at an angle between them. Determine the angle of inclination.
(Solved)
5 images are formed when two mirrors are inclined at an angle between them. Determine the angle of inclination.
Date posted:
May 31, 2019
.
Answers (1)
-
A stone of mass 450g is rotated in a vertical circle at 3 revolutions per second. If the string has a length of 1.5m,
determine:
(i) the...
(Solved)
A stone of mass 450g is rotated in a vertical circle at 3 revolutions per second. If the string has a length of 1.5m,
determine:
(i) the linear velocity
(ii) The tension of the string at positions A and B.
Date posted:
May 31, 2019
.
Answers (1)
-
Two equal masses travel towards each other on a frictionless air track at speeds of 60cm/s and 40cm /s. They stick together on impact.
What is...
(Solved)
Two equal masses travel towards each other on a frictionless air track at speeds of 60cm/s and 40cm /s. They stick together on impact.

What is the velocity of the masses after impact?
Date posted:
May 31, 2019
.
Answers (1)
-
The graph below shows the variation of force with distance for a body being towed.
Calculate the total work done on the body.
(Solved)
The graph below shows the variation of force with distance for a body being towed.
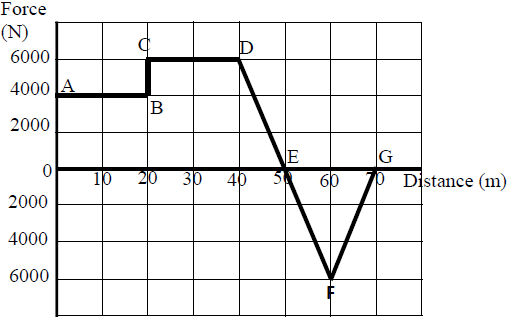
Calculate the total work done on the body.
Date posted:
May 31, 2019
.
Answers (1)
-
Two gear wheel have a 80 teeth (driven) and 20 teeth (driving) and lock with each other. They are fastened on axles of
equal diameters such...
(Solved)
Two gear wheel have a 80 teeth (driven) and 20 teeth (driving) and lock with each other. They are fastened on axles of
equal diameters such that a weight of 150N attached to a string round one axle will just raise 450N on the other axle.
Calculate
(i) M.A
(ii) V.R
(iii) Efficiency of the machine.
Date posted:
May 31, 2019
.
Answers (1)