Increase in temp leads to increase in K. energy of particles increasing their collision making the pressure to increase.
Kavungya answered the question on June 3, 2019 at 07:32
- A uniform metre rule pivoted at it’s 15.0cm mark is balanced by a 2N weight suspended at the 5.0cm mark. Determine the mass of the...(Solved)
A uniform metre rule pivoted at it’s 15.0cm mark is balanced by a 2N weight suspended at the 5.0cm mark. Determine the mass of the metre rule.
Date posted: June 3, 2019. Answers (1)
- On the Cartesian plan below sketch a graph to show how the density of water varies from O0C to 100C.(Solved)
On the Cartesian plan below sketch a graph to show how the density of water varies from O0C to 100C.
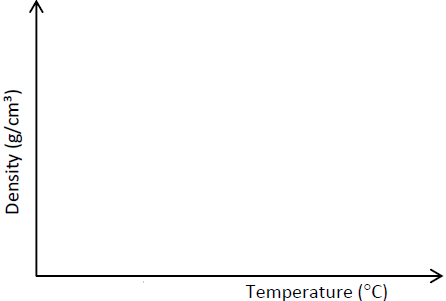
Date posted: June 3, 2019. Answers (1)
- Chalk is denser than air. Explain why chalk dust floats in air.(Solved)
Chalk is denser than air. Explain why chalk dust floats in air.
Date posted: June 3, 2019. Answers (1)
- The barometric height in a town is 65cmHg. Given that the standard atmospheric pressure is 76cmHg and the density of mercy is 13600kg/m³, determine the...(Solved)
The barometric height in a town is 65cmHg. Given that the standard atmospheric pressure is 76cmHg and the density of mercy is 13600kg/m³, determine the attitude of the town. (Density of air is 1.25kg/m³).
Date posted: June 3, 2019. Answers (1)
- A burette has an initial reading of 22.5cm³. Determine the final reading after liquid of volume of 11.3cm³. To removal from the burette.(Solved)
A burette has an initial reading of 22.5cm³. Determine the final reading after liquid of volume of 11.3cm³. To removal from the burette.
Date posted: June 3, 2019. Answers (1)
- The Y-gain of a Cathode Ray Oscilloscope (C.R.O.) is set at 5 volts per division. A 20Vd.c. input is applied to the Y-plates and causes...(Solved)
The Y-gain of a Cathode Ray Oscilloscope (C.R.O.) is set at 5 volts per division. A 20Vd.c. input is applied to the Y-plates and causes a deflection of the bright spot on the screen. Show on the screen below the new position of the bright spot.
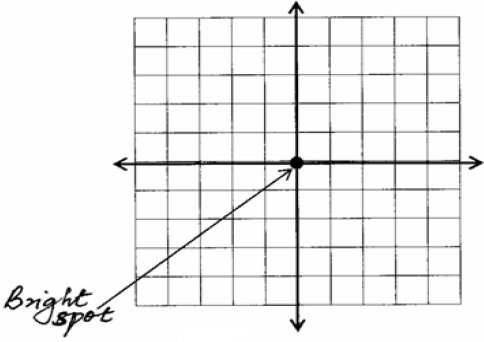
Date posted: June 3, 2019. Answers (1)
- Describe how cathode rays are produced in a cathode ray oscilloscope.(Solved)
Describe how cathode rays are produced in a cathode ray oscilloscope.
Date posted: June 3, 2019. Answers (1)
- The cathode ray tube (C.R.T.) of a television uses magnetic fields for the deflection of the cathode rays.
(i) What are cathode rays?
(ii) Explain why...(Solved)
The cathode ray tube (C.R.T.) of a television uses magnetic fields for the deflection of the cathode rays.
(i) What are cathode rays?
(ii) Explain why magnetic fields are preferred to electric fields in a television tube.
Date posted: June 3, 2019. Answers (1)
- A radiation falls on a photosensitive material. State how the following changes affect the emitted photoelectrons:
(i) increase in intensity of incident radiation.
(ii) increase in...(Solved)
A radiation falls on a photosensitive material. State how the following changes affect the emitted photoelectrons:
(i) increase in intensity of incident radiation.
(ii) increase in the frequency of incident radiation.
Date posted: June 3, 2019. Answers (1)
- Plane water wavefronts are incident onto a concave barrier as shown in the figure below.Show on the same diagram the nature of the reflected wavefronts.(Solved)
Plane water wavefronts are incident onto a concave barrier as shown in the figure below.
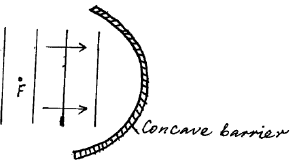
Show on the same diagram the nature of the reflected wavefronts.
Date posted: June 3, 2019. Answers (1)
- A vibrator is used to generate water waves in a ripple tank. It is observed that the distance between the first crest and the mid-point...(Solved)
A vibrator is used to generate water waves in a ripple tank. It is observed that the distance between the first crest and the mid-point to the fifth trough is 237.5cm.
The waves travel 224.0cm in 6.0 seconds.

Determine:
(i) the wavelength of the waves.
(ii) the speed of the waves.
(iii) the frequency of the vibrator.
Date posted: June 3, 2019. Answers (1)
- The figure below shows water wave fronts approaching a boundary between a shallow and a deep region. The
speed of waves in the shallow region is...(Solved)
The figure below shows water wave fronts approaching a boundary between a shallow and a deep region. The
speed of waves in the shallow region is less than in the deep region.
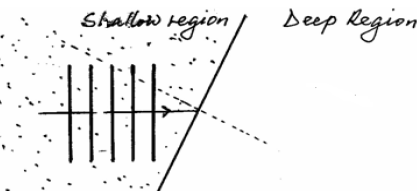
On the same diagram, complete the figure to show the wavefronts after crossing the boundary.
Date posted: June 3, 2019. Answers (1)
- The figure below shows a parabolic surface with a source of light placed at its focal point F.
Draw rays to show reflection from the surface...(Solved)
The figure below shows a parabolic surface with a source of light placed at its focal point F.
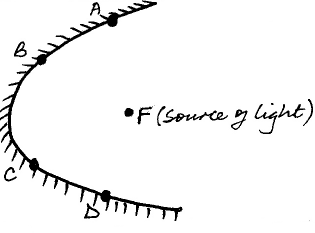
Draw rays to show reflection from the surface when rays from the source strike the surface at points A, B, C and D.
Date posted: June 3, 2019. Answers (1)
- An immersion heater rated 2.5KW is used in a home continuously for 50 minutes per day in a month of 30 days. Given that electricity...(Solved)
An immersion heater rated 2.5KW is used in a home continuously for 50 minutes per day in a month of 30 days. Given that electricity costs Kshs.7.60 per unit (1 unit = 1kwh)), calculate:
(i) the number of KW transformed in the month.
(ii) the number of units (kwh) consumed in the month.
(iii) the total cost of the electric power consumed in the month given that there is a fixed charge of Kshs.150.00.
Date posted: June 3, 2019. Answers (1)
- The north pole of a magnet is moved away from a metallic ring, as shown in the figure below. Show the direction of current induced...(Solved)
The north pole of a magnet is moved away from a metallic ring, as shown in the figure below. Show the direction of current induced in the ring.
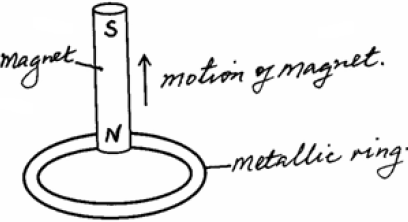
Date posted: June 3, 2019. Answers (1)
- State:
(a) one application of ultraviolet radiation.
(b) one detector of the radiation in (a) above.(Solved)
State:
(a) one application of ultraviolet radiation.
(b) one detector of the radiation in (a) above.
Date posted: June 3, 2019. Answers (1)
- A dielectric material X is now inserted between the plates, as shown below.
State how each of the following is affected by insertion of material:
(a) the...(Solved)
A dielectric material X is now inserted between the plates, as shown below.
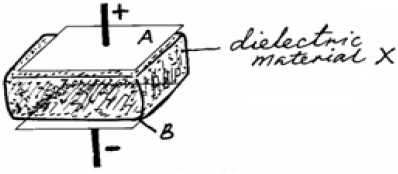
State how each of the following is affected by insertion of material:
(a) the potential difference (p.d) across the plates;
(b) capacitance of the set up.
Date posted: June 3, 2019. Answers (1)
- A sample of Iodine-131 contains 1000g. If the half-life of Iodine-131 is 8 days, how much of the sample will remain undecayed after 40 days?(Solved)
A sample of Iodine-131 contains 1000g. If the half-life of Iodine-131 is 8 days, how much of the sample will remain undecayed after 40 days?
Date posted: June 3, 2019. Answers (1)
- Sketch the Current-Voltage characteristic for a forward -biased diode.(Solved)
Sketch the Current-Voltage characteristic for a forward -biased diode.
Date posted: June 3, 2019. Answers (1)
- The figure below shows a thick copper conductor placed between two poles of a strong magnet. The wire is free to swing in between the...(Solved)
The figure below shows a thick copper conductor placed between two poles of a strong magnet. The wire is free to swing in between the poles.
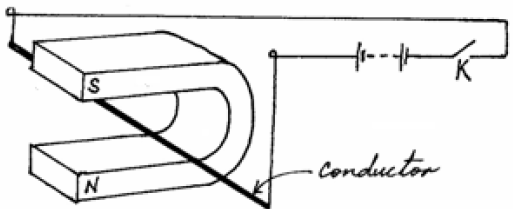
(a) Indicate on the same diagram the direction in which the conductor swings when the switch K is closed.
(b) State one change that can be made on the set up so that the direction of swing of the conductor is reversed.
Date posted: June 3, 2019. Answers (1)