-
State the difference between the temperature measured in Kelvin scale and Celsius scale.
(Solved)
State the difference between the temperature measured in Kelvin scale and Celsius scale.
Date posted:
June 3, 2019
.
Answers (1)
-
A steel cylinder of capacity 0.5m³ contains nitrogen at a pressure of 30,000Pa when the temperature is 270C. What will be the pressure of nitrogen...
(Solved)
A steel cylinder of capacity 0.5m³ contains nitrogen at a pressure of 30,000Pa when the temperature is 270C. What will be the pressure of nitrogen if it is allowed to flow into another cylinder of capacity 9.5m³ with the temperature reduced to -230C?
Date posted:
June 3, 2019
.
Answers (1)
-
The figure below shows a set-up that may be used to verify pressure law.
(i) State the measurements that should be taken in the experiment.
(ii) Explain...
(Solved)
The figure below shows a set-up that may be used to verify pressure law.

(i) State the measurements that should be taken in the experiment.
(ii) Explain how the measurements in (i) above may be used to verify pressure law.
Date posted:
June 3, 2019
.
Answers (1)
-
A uniform metre rule pivoted at it’s 15.0cm mark is balanced by a 2N weight suspended at the 5.0cm mark. Determine the mass of the...
(Solved)
A uniform metre rule pivoted at it’s 15.0cm mark is balanced by a 2N weight suspended at the 5.0cm mark. Determine the mass of the metre rule.
Date posted:
June 3, 2019
.
Answers (1)
-
Chalk is denser than air. Explain why chalk dust floats in air.
(Solved)
Chalk is denser than air. Explain why chalk dust floats in air.
Date posted:
June 3, 2019
.
Answers (1)
-
The barometric height in a town is 65cmHg. Given that the standard atmospheric pressure is 76cmHg and the density of mercy is 13600kg/m³, determine the...
(Solved)
The barometric height in a town is 65cmHg. Given that the standard atmospheric pressure is 76cmHg and the density of mercy is 13600kg/m³, determine the attitude of the town. (Density of air is 1.25kg/m³).
Date posted:
June 3, 2019
.
Answers (1)
-
A burette has an initial reading of 22.5cm³. Determine the final reading after liquid of volume of 11.3cm³. To removal from the burette.
(Solved)
A burette has an initial reading of 22.5cm³. Determine the final reading after liquid of volume of 11.3cm³. To removal from the burette.
Date posted:
June 3, 2019
.
Answers (1)
-
Plane water wavefronts are incident onto a concave barrier as shown in the figure below.Show on the same diagram the nature of the reflected wavefronts.
(Solved)
Plane water wavefronts are incident onto a concave barrier as shown in the figure below.
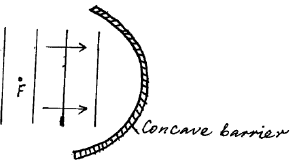
Show on the same diagram the nature of the reflected wavefronts.
Date posted:
June 3, 2019
.
Answers (1)
-
A vibrator is used to generate water waves in a ripple tank. It is observed that the distance between the first crest and the mid-point...
(Solved)
A vibrator is used to generate water waves in a ripple tank. It is observed that the distance between the first crest and the mid-point to the fifth trough is 237.5cm.
The waves travel 224.0cm in 6.0 seconds.

Determine:
(i) the wavelength of the waves.
(ii) the speed of the waves.
(iii) the frequency of the vibrator.
Date posted:
June 3, 2019
.
Answers (1)
-
A sample of Iodine-131 contains 1000g. If the half-life of Iodine-131 is 8 days, how much of the sample will remain undecayed after 40 days?
(Solved)
A sample of Iodine-131 contains 1000g. If the half-life of Iodine-131 is 8 days, how much of the sample will remain undecayed after 40 days?
Date posted:
June 3, 2019
.
Answers (1)
-
The figure below shows a thick copper conductor placed between two poles of a strong magnet. The wire is free to swing in between the...
(Solved)
The figure below shows a thick copper conductor placed between two poles of a strong magnet. The wire is free to swing in between the poles.
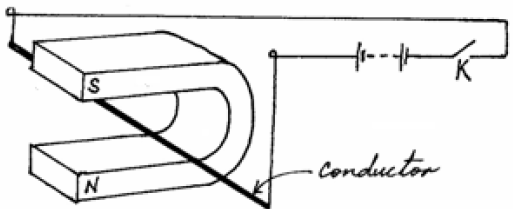
(a) Indicate on the same diagram the direction in which the conductor swings when the switch K is closed.
(b) State one change that can be made on the set up so that the direction of swing of the conductor is reversed.
Date posted:
June 3, 2019
.
Answers (1)
-
A girl standing 120m away from a tall building claps her hands and hears an echo 0.75s later. Determine the speed of sound in air...
(Solved)
A girl standing 120m away from a tall building claps her hands and hears an echo 0.75s later. Determine the speed of sound in air at this place.
Date posted:
June 3, 2019
.
Answers (1)
-
The figure below shows an incomplete circuit of an electromagnet.
Complete the circuit by drawing the windings on the two arms of the core so that...
(Solved)
The figure below shows an incomplete circuit of an electromagnet.

Complete the circuit by drawing the windings on the two arms of the core so that A and B are both north poles when the switch S is closed. Indicate the direction of the current of the windings drawn.
Date posted:
June 3, 2019
.
Answers (1)
-
A current of 0.8A flows through an electric circuit. Determine the quantity of charge that passes a point in the circuit in 6 minutes.
(Solved)
A current of 0.8A flows through an electric circuit. Determine the quantity of charge that passes a point in the circuit in 6 minutes.
Date posted:
June 3, 2019
.
Answers (1)
-
The figure below shows an iron cylinder of length 10cm and uniform cross-section 2cm² suspended
from a spring balance with half of its length immersed in...
(Solved)
The figure below shows an iron cylinder of length 10cm and uniform cross-section 2cm² suspended
from a spring balance with half of its length immersed in paraffin oil of density 0.8gcmˉ³.

(i) Show on the diagram, the forces acting on the iron cylinder.
(ii) If the density of iron is 7.5gmˉ³ determine.
(i) the weight of the iron cylinder.
(ii) the reading of the spring balance.
Date posted:
June 3, 2019
.
Answers (1)
-
Draw a clear labeled diagram of a common hydrometer which is suitable for measuring the densities of liquid varying between 1.0 and 1.2 g/cm³.
(Solved)
Draw a clear labeled diagram of a common hydrometer which is suitable for measuring the densities of liquid varying between 1.0 and 1.2 g/cm³.
Date posted:
June 3, 2019
.
Answers (1)
-
A trolley of height 0.2m moving on a horizontal bench of height 3.2m strikes a barrier at the edge of the bench. The object on...
(Solved)
A trolley of height 0.2m moving on a horizontal bench of height 3.2m strikes a barrier at the edge of the bench. The object on top of the trolley flies off on impact and lands on the ground 2.5m from the edge of the bench as shown in the figure below.

Use this information to answer the questions that follow.
(i) Give a reason why the object on the trolley flies off on impact.
(ii) Determine the time taken by the object to reach the ground.
Date posted:
June 3, 2019
.
Answers (1)
-
A stone is thrown vertically upwards from an edge of a platform. Eventually the stone lands without bouncing, on the ground below the platform. Taking...
(Solved)
A stone is thrown vertically upwards from an edge of a platform. Eventually the stone lands without bouncing, on the ground below the platform. Taking the upward velocity to be positive, sketch the velocity-time graph of the motion of the stone.
Date posted:
June 1, 2019
.
Answers (1)
-
The mercury column in a barometer is 760mm high. Taking the density of mercury to be 13.6g/cm³, calculate the atmospheric pressure in N/m².
(Solved)
The mercury column in a barometer is 760mm high. Taking the density of mercury to be 13.6g/cm³, calculate the atmospheric pressure in N/m².
Date posted:
June 1, 2019
.
Answers (1)
-
An object weighs 49N on earth where acceleration due to gravity is 9.8N/kg. Find the acceleration due to gravity on another planet where the same...
(Solved)
An object weighs 49N on earth where acceleration due to gravity is 9.8N/kg. Find the acceleration due to gravity on another planet where the same object weighs 40.5N.
Date posted:
June 1, 2019
.
Answers (1)