- A test tube of mass 10g and uniform cross-sectional area 4cm2 is partly filled with lead shots and floats vertically in water with 5cm of...(Solved)
A test tube of mass 10g and uniform cross-sectional area 4cm2 is partly filled with lead shots and floats vertically in water with 5cm of its length submerged.
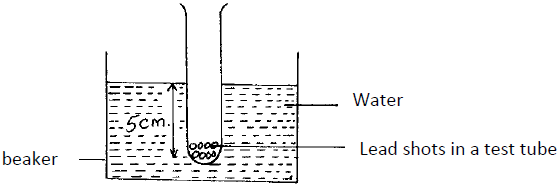
Find the:-
(i) Mass of the lead shots.
(ii) Length of the test tube that would be submerged in a liquid of density 0.75g/cm3.
Date posted: June 3, 2019. Answers (1)
- The figure below shows a rectangular buoy of mass 4000kg tethered to the sea-bed by a wire. The dimensions are 4m x 1.5m x 2.2m.
Calculate...(Solved)
The figure below shows a rectangular buoy of mass 4000kg tethered to the sea-bed by a wire. The dimensions are 4m x 1.5m x 2.2m.
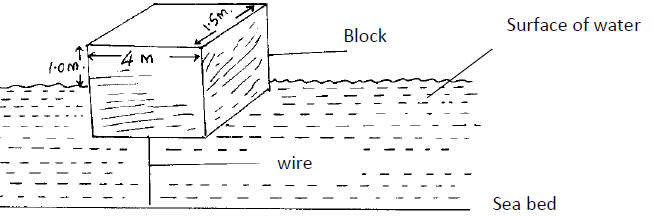
Calculate the :-
(i) Weight of sea water displaced by the buoy (density of sea water = 1100kg/m3)
(ii) Upward force exerted on the buoy by the water.
(iii) Tension in the wire
Date posted: June 3, 2019. Answers (1)
- The effort piston of a hydraulic machine is of radius 2.8 cm, while that of the load piston is of radius 14cm. The machine raises...(Solved)
The effort piston of a hydraulic machine is of radius 2.8 cm, while that of the load piston is of radius 14cm. The machine raises a load of 120 kg at a constant velocity through 2.5m. If the machine has an efficiency of 80%, find:-
(i) the velocity ratio of the hydraulic machine.
(ii) The mechanical advantage of the hydraulic machine.
(iii) The effort needed to raise the load.
Date posted: June 3, 2019. Answers (1)
- A quantity of air occupied 500cm3 at 150C when the pressure was 76 cm. Hg. At what temperature would it occupy 43 if the pressure...(Solved)
A quantity of air occupied 500cm3 at 150C when the pressure was 76 cm. Hg. At what temperature would it occupy 43 if the pressure was 85cmHg?
Date posted: June 3, 2019. Answers (1)
- Sea water of density 1.04g/cm3 is being pumped into a tank through a pipe of uniform cross-sectional area of 3.142cm2. If the speed of water...(Solved)
Sea water of density 1.04g/cm3 is being pumped into a tank through a pipe of uniform cross-sectional area of 3.142cm2. If the speed of water in the pipe is 5m/s, determine the mass flux in S.I unit.
Date posted: June 3, 2019. Answers (1)
- A spherical ball bearing of mass 0.0024 kg is held between the anvil and spindle of a micrometer screw gauge. The reading on the gauge...(Solved)
A spherical ball bearing of mass 0.0024 kg is held between the anvil and spindle of a micrometer screw gauge. The reading on the gauge when the jaws are closed without anything in between is 0.11mm. Use this information and the position of the scale in the figure below to answer the questions (a) and (b) below:

a) What is the diameter of the ball bearing?
b) Find the density of the ball bearing correct to 3 significant figures
Date posted: June 3, 2019. Answers (1)
- Draw a circuit of showing full-wave rectification using a bridge rectifier.(Solved)
Draw a circuit of showing full-wave rectification using a bridge rectifier.
Date posted: June 3, 2019. Answers (1)
- A piece of phosphorous crystal is added in the structure of silicon, state the minority charge carrier of the semiconductor formed.(Solved)
A piece of phosphorous crystal is added in the structure of silicon, state the minority charge carrier of the semiconductor formed.
Date posted: June 3, 2019. Answers (1)
- The figure below shows a P-N junction diode connected to a dry cell.plot the I.V characteristic of diode.(Solved)
The figure below shows a P-N junction diode connected to a dry cell.

plot the I.V characteristic of diode.
Date posted: June 3, 2019. Answers (1)
- An certain metal surface has a work function of 2.04 x 10-19J. Calculate the maximum kinetic energy in electron volt of the liberated elections when...(Solved)
An certain metal surface has a work function of 2.04 x 10-19J. Calculate the maximum kinetic energy in electron volt of the liberated elections when the metal is illuminated by light of wavelength 4.5 x 10-7m.
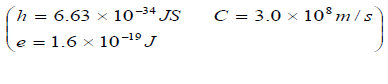
Date posted: June 3, 2019. Answers (1)
- For a certain radioactive material, the average count-rate is found to be 82 counts per second. After 210 seconds, the count rate had dropped by...(Solved)
For a certain radioactive material, the average count-rate is found to be 82 counts per second. After 210 seconds, the count rate had dropped by 63 counts per second. The average background count-rate remained constant at 10 counts per second. What is the half-life of the material?
Date posted: June 3, 2019. Answers (1)
- When the time-base is off, the length of the line on a CRO screen is 4cm peak to peak for an AC voltage
400V. Determine the...(Solved)
When the time-base is off, the length of the line on a CRO screen is 4cm peak to peak for an AC voltage
400V. Determine the sensitivity setting.
Date posted: June 3, 2019. Answers (1)
- The figure below shows the wiring in a modern main appliance.
(i) Identify the colour of wires Y and Z.
(ii) A cooker rated 2.0KW was...(Solved)
The figure below shows the wiring in a modern main appliance.

(i) Identify the colour of wires Y and Z.
(ii) A cooker rated 2.0KW was operated for 35 minutes each day for 30 days.
If the cost of each unit is Sh.12.5 Calculate the cost of electricity used.
Date posted: June 3, 2019. Answers (1)
- State the adjustments that can be made on a d.c generator to produce alternating current.(Solved)
State the adjustments that can be made on a d.c generator to produce alternating current.
Date posted: June 3, 2019. Answers (1)
- A transformer is used on a 240V a.c supply to deliver 12A at 120V to a heating coil. If 20% energy is lost at the...(Solved)
A transformer is used on a 240V a.c supply to deliver 12A at 120V to a heating coil. If 20% energy is lost at the transformer, calculate the current in the primary coil.
Date posted: June 3, 2019. Answers (1)
- The diagram below shows an induction coil used to produce sparks.
(i) Name the part labelled C.
(ii) Explain why the part labelled A is thicker than...(Solved)
The diagram below shows an induction coil used to produce sparks.

(i) Name the part labelled C.
(ii) Explain why the part labelled A is thicker than C.
(iii) State the purpose of part labelled B.
Date posted: June 3, 2019. Answers (1)
- The diagram below represents an electrical circuit with switches S1 and S2 open.
Switch S1 is closed and the circuit, left undisturbed for a few minutes.
Determine:
(i)...(Solved)
The diagram below represents an electrical circuit with switches S1 and S2 open.

Switch S1 is closed and the circuit, left undisturbed for a few minutes.
Determine:
(i) the potential difference between W and X.
(ii) the magnitude of charge on each plate of capacitor C1 in SI units.
(iii) the potential difference between Z and Y.
Date posted: June 3, 2019. Answers (1)
- The figure below shows a net work of resistors connected together in a circuit.
The voltmeter reads 3.0V when the switch is open and 2.4V when...(Solved)
The figure below shows a net work of resistors connected together in a circuit.
The voltmeter reads 3.0V when the switch is open and 2.4V when switch is closed.

Given that the ammeter reads 0.6A.
Determine:
(i) the net e.m.f of the battery.
(ii) the internal resistance of each cell.
(iii) the potential drop across the 3 resistor.
Date posted: June 3, 2019. Answers (1)
- A ray of light is incident on a glass-water interface making an angle of 350 with the boundary as shown below.
Calculate the angle of refraction...(Solved)
A ray of light is incident on a glass-water interface making an angle of 350 with the boundary as shown below.

Calculate the angle of refraction (take refractive of glass and water as 2/3 and 4/3 respectively.
Date posted: June 3, 2019. Answers (1)
- In a compound microscope, the focal length of the objective lens is 3.0cm and that of the eye piece is 3.2cm and they are placed...(Solved)
In a compound microscope, the focal length of the objective lens is 3.0cm and that of the eye piece is 3.2cm and they are placed 10.0cm apart. An object is placed 5cm from the objective lens. Use the lens formular. In turn for each lens to find the position of the final image.
Date posted: June 3, 2019. Answers (1)