To the mixture,add methyl benzene.Iodine will dissolve.
Filter and crystallize to obtain iodine crystals.
Heat the residue to sublime ammonium chloride.
To the residue,add water to dissolve sodium chloride.
otienosam answered the question on June 6, 2019 at 12:02
-
Why is potassium nitrate preferred in the laboratory preparation of nitric(V) acid?
(Solved)
Why is potassium nitrate preferred in the laboratory preparation of nitric(V) acid?
Date posted:
June 4, 2019
.
Answers (1)
-
Give the contrasting difference in chemical properties of chlorine and hydrogen?
(Solved)
Give the contrasting difference in chemical properties of chlorine and hydrogen?
Date posted:
May 24, 2019
.
Answers (1)
-
A volume of 15cm3 of ethane gas was exploded with 50cm3 of oxygen. If both volumes were measured at the same temperature and pressure, calculate...
(Solved)
A volume of 15cm3 of ethane gas was exploded with 50cm3 of oxygen. If both volumes were measured at the same temperature and pressure, calculate the volume of resulting gaseous mixture.
Date posted:
May 24, 2019
.
Answers (1)
-
An element Q has a relative atomic mass of 88. When a current of 0.5A was passed through the fused chloride of Q for 32
minutes...
(Solved)
An element Q has a relative atomic mass of 88. When a current of 0.5A was passed through the fused chloride of Q for 32 minutes and 10 seconds, 0.44g of Q were deposited at cathode. Determine the charge on the ion of Q. (1 Faraday = 9650
Date posted:
May 24, 2019
.
Answers (1)
-
The electronic structures for elements represented by letters A, B, C and D are
A - 2.8.6 B - 2.8.2 C - 2.8.1 D - 2.8.8
a)...
(Solved)
The electronic structures for elements represented by letters A, B, C and D are
A - 2.8.6 B - 2.8.2 C - 2.8.1 D - 2.8.8
a) Select the element which forms :
i) a double charged cation
ii) a soluble carbonate
b) Which element has the shortest atomic radius ?
Date posted:
May 24, 2019
.
Answers (1)
-
Metal S removes oxygen combined with P. Q reacts with an oxide of R and not with an oxide of P. P reacts with cold...
(Solved)
Metal S removes oxygen combined with P. Q reacts with an oxide of R and not with an oxide of P. P reacts with cold water
but Q does not.
a) Which is the most reactive metal ?
b) Which is the least reactive metal ?
c) Arrange the metals in order of reactivity starting with most reactive to the least reactive.
Date posted:
May 24, 2019
.
Answers (1)
-
Below are PH values of some solutions.
i) Which solution is likely to be
I. acidic rain
II. Potassium hydroxide
ii) A basic substance V...
(Solved)
Below are PH values of some solutions.

i) Which solution is likely to be
I. acidic rain
II. Potassium hydroxide
ii) A basic substance V reacted with both solutions Y and X. What is the nature of V.
iii) Identify two substances that show these characteristics in question (ii) above.
Date posted:
May 24, 2019
.
Answers (1)
-
The diagram below is a section of a model of the structure of element T.
a) State the type of bonding that exist in T.
b) In...
(Solved)
The diagram below is a section of a model of the structure of element T.
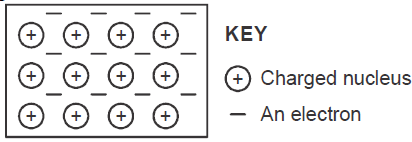
a) State the type of bonding that exist in T.
b) In which group of the periodic table does element T belong ? Give a reason.
Date posted:
May 24, 2019
.
Answers (1)
-
20cm3 of an unknown gas Q takes 12.6 seconds to pass through small orifice. 10cm3 of oxygen gas takes 11.2 seconds to diffuse through the...
(Solved)
20cm3 of an unknown gas Q takes 12.6 seconds to pass through small orifice. 10cm3 of oxygen gas takes 11.2 seconds to
diffuse through the same orifice under the same conditions of temperature and pressure. Calculate the molecular mass of
unknown gas. (O = 16)
Date posted:
May 24, 2019
.
Answers (1)
-
When one mole of lithium has completely reacted what volume of hydrogen would be produced at room temperature? (MGV = 24dm³, Li=7)
(Solved)
When one mole of lithium has completely reacted what volume of hydrogen would be produced at room temperature? (MGV = 24dm³, Li=7)
Date posted:
May 24, 2019
.
Answers (1)
-
When sulphur is heated in a boiling tube in the absence of air, the yellow crystals melts into a golden yellow mobile liquid at
113°C. The...
(Solved)
When sulphur is heated in a boiling tube in the absence of air, the yellow crystals melts into a golden yellow mobile liquid at
113°C. The liquid changes at 180°C into a dark brown liquid that is very viscous. More heating at 400°C produces a brown
less viscous liquid.
a) Draw the molecular structure of sulphur in the yellow liquid.
b) Explain why the molten liquid becomes viscous.
Date posted:
May 23, 2019
.
Answers (1)
-
Use dots (•) and cross (×) diagrams to draw bond in:
a) Al2Cl6 (Al = 13, Cl=17)
b) Al2O3 (Al = 13, O = 8)
(Solved)
Use dots (•) and cross (×) diagrams to draw bond in:
a) Al2Cl6 (Al = 13, Cl=17)
b) Al2O3 (Al = 13, O = 8)
Date posted:
May 23, 2019
.
Answers (1)
-
Calculate the number of molecules of water of crystallization in oxalic acid crystals, H2C2O4.nH2O given that 5g of the
crystals were made upto 250cm³ of this...
(Solved)
Calculate the number of molecules of water of crystallization in oxalic acid crystals, H2C2O4.nH2O given that 5g of the
crystals were made upto 250cm³ of this solution. 25.0cm³ of this solution required 15.9cm³ of 0.5M sodium hydroxide to
neutralise it (H=1, C=12, O=16).
Date posted:
May 23, 2019
.
Answers (1)
-
Nylon 6, 6 is a condensation polymer whose structure is as follows.
Draw the structures of the monomers in nylon 6, 6
(Solved)
Nylon 6, 6 is a condensation polymer whose structure is as follows.

Draw the structures of the monomers in nylon 6, 6
Date posted:
May 23, 2019
.
Answers (1)
-
Magnesium reacts as shown below.
a) Identify gas X.
b) Between wet sand and magnesium ribbon, which one should be heated first? Explain.
(Solved)
Magnesium reacts as shown below.


a) Identify gas X.
b) Between wet sand and magnesium ribbon, which one should be heated first? Explain.
Date posted:
May 23, 2019
.
Answers (1)
-
The diagram below was used to electrolyse magnesium sulphate solution
a) Write half equation at electrode. P,Q
b) State what happens to the concentration of the...
(Solved)
The diagram below was used to electrolyse magnesium sulphate solution
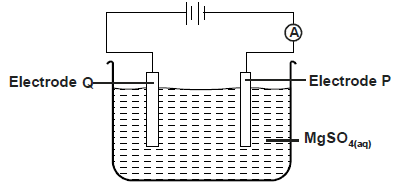
a) Write half equation at electrode. P,Q
b) State what happens to the concentration of the electrolyte after electrolysis process.
Date posted:
May 23, 2019
.
Answers (1)
-
Use the diagram below to answer the questions that follow.
a) Why is sodium hydroxide solution preferred to water in the above set-up.
b) What modification should...
(Solved)
Use the diagram below to answer the questions that follow.
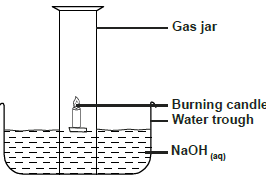
a) Why is sodium hydroxide solution preferred to water in the above set-up.
b) What modification should be made to the above set-up if percentage of oxygen used in air should be determined
c) Name the main component of air not used in the above set-up.
Date posted:
May 23, 2019
.
Answers (1)
-
0.63g of lead powder were dissolved in excess nitric (V) acid to form lead (II) nitrate solution. All the lead (II) nitrate was
then reacted with...
(Solved)
0.63g of lead powder were dissolved in excess nitric (V) acid to form lead (II) nitrate solution. All the lead (II) nitrate was
then reacted with sodium sulphate solution.
a) Write an ionic equation for the reaction between sodium sulphate solution and lead (II) nitrate solution.
b) Determine the mass of the lead salt formed in the reaction in (a) above
(Pb = 207, S = 32, O = 16)
Date posted:
May 23, 2019
.
Answers (1)
-
Study the diagram below then use it to answer the questions that follow.
a) Draw the wooden splint at the end of the experiment. If it...
(Solved)
Study the diagram below then use it to answer the questions that follow.

a) Draw the wooden splint at the end of the experiment. If it was slipped then removed.
b) Explain the appearance of the wooden splint in (a) above.
Date posted:
May 23, 2019
.
Answers (1)
-
The diagram below shows acidic and basic oxides fit in a general family of oxides.
a) State the name given to the type of oxides that...
(Solved)
The diagram below shows acidic and basic oxides fit in a general family of oxides.

a) State the name given to the type of oxides that would be placed in the shaded region.
b) Name two oxides that could be placed on the shaded region.
Date posted:
May 22, 2019
.
Answers (1)