a)Energy change that occurs when one mole of gaseous ions become hydrated//form weak
bonds with water molecules
b)?Hsoln= Lattice energy + Hydration energy
=+2237+(-1891)+2(-384)
= -422 kJ mol-1
Githiari answered the question on September 13, 2017 at 02:15
- Study the equation below and answer the questions that follow C6H14 + Cl2--->C6H13Cl + HCl(Solved)
(i) State the condition under which this reaction occurs.(1 mark)
(ii) Give the general name of this type of reaction. (1 mark)
Date posted: September 13, 2017. Answers (1)
- Nitrogen gas can be prepared in the laboratory using a mixture of ammonium chloride solution and sodium nitrite solution.(Solved)
(a) The reaction occurs in two steps. State the two steps in the correct order. (2 marks)
(b) State two applications nitrogen. (1 mark)
Date posted: September 13, 2017. Answers (1)
- A mixture consists of sulphur powder and iron filings.(i) Describe how to obtain sulphur from the mixture using methylbenzene. (Solved)
(i) Describe how to obtain sulphur from the mixture using methylbenzene. (3 marks)
(ii) Is the mixture homogeneous or heterogeneous? Explain. (2 marks)
Date posted: September 13, 2017. Answers (1)
- What is the importance of the shape of a conical flask?(Solved)
What is the importance of the shape of a conical flask?
Date posted: September 13, 2017. Answers (1)
- Using dot and cross to represent electrons draw a diagram to illustrate bonding in the sulphide of a substance with a pH of 2.2(Solved)
Using dot and cross to represent electrons draw a diagram to illustrate bonding in the sulphide of a substance with a pH of 2.2
Date posted: September 10, 2017. Answers (1)
- The graph below shows the mass of a radioactive isotope plotted against time.
(Solved)
The graph below shows the mass of a radioactive isotope plotted against time.
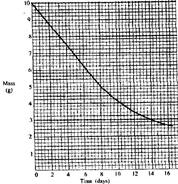
(a) Using the graph, determine the half-life of the isotope.
(b) Calculate the mass of the isotope present after 32 days.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and use it to answer the questions that follow. (Solved)
Study the flow chart below and use it to answer the questions that follow.
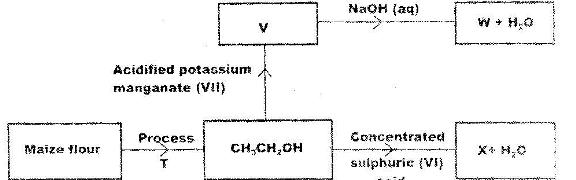
(a) Name process T.
(b) Give the formula of W.
(c) State two uses of X.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow. (Solved)
Study the flow chart below and answer the questions that follow.
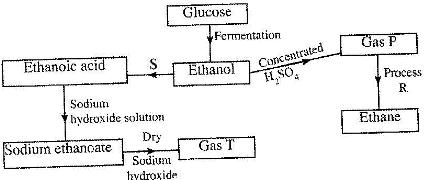
(i) State the conditions necessary for fermentation of glucose to take place.
(ii) State the reagent that can be used to carry out process S.
(iii) Identify gases P and T.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and answer the questions that follows.
(Solved)
Study the flow chart below and answer the questions that follows.
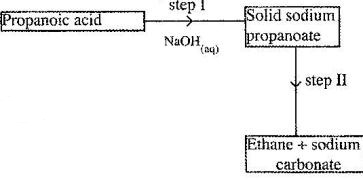
(a) Name the process in step I.
(b) Identify the reagent in step II.
(c) Give one use of ethane.
Date posted: June 7, 2017. Answers (1)
- The table below shows the relative molecular masses and boiling points of pentane and ethanoic acid. (Solved)
The table below shows the relative molecular masses and boiling points of pentane and ethanoic acid.
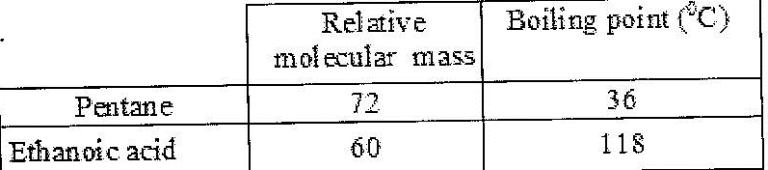
Explain the large difference in boiling point between ethanoic acid and pentane.
Date posted: June 7, 2017. Answers (1)
- The structure below represents a type of a cleansing agent. (Solved)
The structure below represents a type of a cleansing agent.
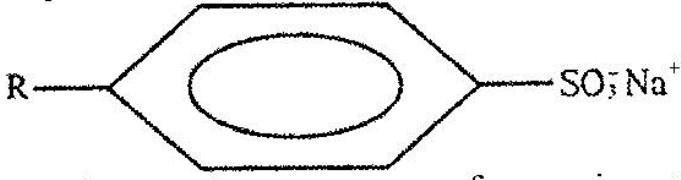
Describe how the cleansing agent removes grease from a piece of cloth.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and use it to answer the questions that follow: (Solved)
Study the flow chart below and use it to answer the questions that follow:
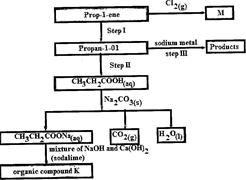
(i) Identify the organic compound K.
(ii) Write the formula of M
(iii) Give one reagent that can be used in:
(I) Step I; (II) step II
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow. (Solved)
Study the flow chart below and answer the questions that follow.
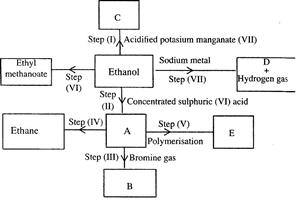
(i) (I) What is observation will be made in step I?
(II) Describe a chemical test that can be carried out to show the identity of compound C.
(ii) Give the names of the following: I. E II. Substance D.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow. (Solved)
Study the flow chart below and answer the questions that follow.
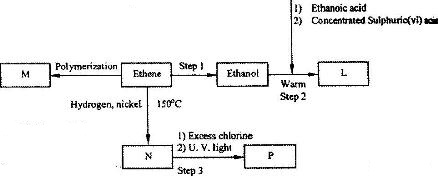
(i) Name the compounds:
(I) L
(II) N
(ii) Give the reagent and the condition used in step 1.
(iii) State the type of reaction that takes place in:
(I) Step 2; (II) step 3;
Date posted: June 7, 2017. Answers (1)
- Describe a chemical test that can be carried out in order to distinguish between. (Solved)
Describe a chemical test that can be carried out in order to distinguish between.
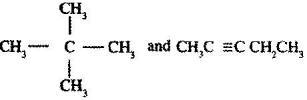
Date posted: June 7, 2017. Answers (1)
- Give the names of the following compounds:(Solved)
Give the names of the following compounds:
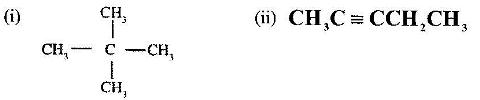
Date posted: June 7, 2017. Answers (1)
- Use the flow chart below to answer the questions that follow. (Solved)
Use the flow chart below to answer the questions that follow.
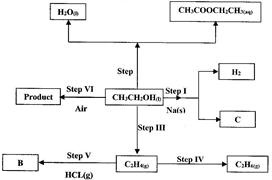
(i) Name:
(I) The type of reaction that occurs in step II;
(II) Substance B.
(ii) Give the formula of substance C.
Date posted: June 7, 2017. Answers (1)
- The table below gives the formula of four compounds L, M, N and P. (Solved)
The table below gives the formula of four compounds L, M, N and P.
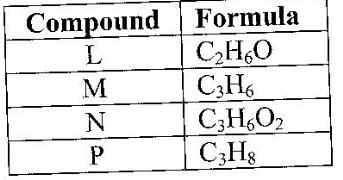
Giving a reason in each case, select the letter which represents a compound that:
(i) Decolorizes bromine in the absence of UV light.
(ii) Gives effervescence when reacted with aqueous sodium carbonate.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and answer the questions that follow. (Solved)
Study the flow chart below and answer the questions that follow.
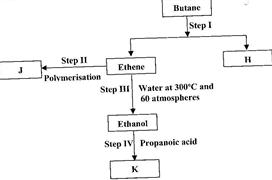
(i) State the conditions for the reaction in Step I to occur.
(ii) Identify substance J.
(iii) State one disadvantage of the continued use of substance such as J.
Date posted: June 7, 2017. Answers (1)
- The structure below represents a sweet smelling compound.(Solved)
The structure below represents a sweet smelling compound.
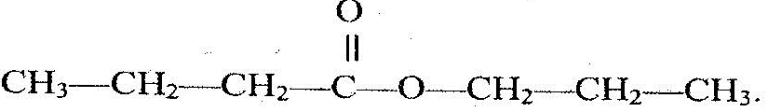
Give the name of two organic compounds that can be used to prepare this compound in the laboratory.
Date posted: June 7, 2017. Answers (1)