a)The amount of substance discharged at the electrodes is directly proportional to the quantity of electricity passed.
b)(Au =197)
Au3+ (aq) + 3e- ------------>Au(s)
1 F=96500
3F= 96500×3=289500 C
289500=197
=2.5
(2.5 ×289500)/197=3673.86 C
Q=It=3673.86=2.5×t
t=(1469.59)/60
=24.49 mins
c)i.Al(s) // Al(aq) // Cu+(aq // Cu (s)
ii)Anode- Al(s)------------>Al3+ (aq) +3e-
Cathode - Cu+ + e- ------------->Cu(s)
iii)Emf= EReduced - EOxidised
0.52- -1.66= +2.18V
iv)Al(s)+ 3Cu+(aq -------> Al3+(aq) + 3Cu (s) +2.18
d)Ag+(aq) + e- --------------->Ag (s)
Githiari answered the question on September 14, 2017 at 20:26
- a)Given that the mass of copper obtained in an o extraction was 210kg, determine the percentage purity of the ore used (copper pyrites) if 810kg...(Solved)
a)Given that the mass of copper obtained in an o extraction was 210kg, determine the percentage purity of the ore used (copper pyrites) if 810kg of it was fed to the 1st roasting furnace. (Cu = 63.5, Fe = 56, S = 32.0)
b)Give 2 effects that the process above could have on the environment.
Date posted: September 14, 2017. Answers (1)
- A student found a bottle containing CH3CH2COOCH3.
(i)Name the process of formation of the substance above
ii)Identify the two substances from which the substance above is derived...(Solved)
A student found a bottle containing CH3CH2COOCH3.
(i)Name the process of formation of the substance above
ii)Identify the two substances from which the substance above is derived .
iii)Give one advantage and one disadvantage of using soapless detergent
iv)Explain briefly how the soapless detergents given above may be manufactured
Date posted: September 14, 2017. Answers (1)
- Write down the equation for the reaction between Ethene and hydrogen when equal numbers of moles are used.(Solved)
Write down the equation for the reaction between Ethene and hydrogen when equal numbers of moles are used.
Date posted: September 14, 2017. Answers (1)
- Explain how ethane and ethene can be distinguished by burning(Solved)
Explain how ethane and ethene can be distinguished by burning
Date posted: September 14, 2017. Answers (1)
- a) Explain how a catalyst affects the activation energy during chemical reaction. (b)In an experiment to determine the heat of combustion of methanol. CH3OH, a student...(Solved)
A) Explain how a catalyst affects the activation energy during chemical reaction.
B)In an experiment to determine the heat of combustion of methanol. CH3OH, a student was recorded the following requirement the following data
Volume of water =100cm3
Final temperature of water = 22.00c
Initial temperature of water = 36.00c
Final mass of lamp an methanol = 84.75g
Initial mass of lamp and methanol=85.10g
Density of water = 1 g/cm3
(S.H.C of water = 4.23 g-1K-1)
i)Write an equation for the combustion of methanol
(ii) Calculate:
(a) Number of moles of methanol used in this experiment
(b) The heat change for this experiment
(c) The heat of combustion per mole of methanol
(d) Explain why the molar heat of combustion for methanol obtained above is different from the theoretical value.
(e) State two factors to consider when choosing a fuel.
(f) Outline two disadvantages of using hydrogen as a source of fuel.
Date posted: September 14, 2017. Answers (1)
- a)Using ionic equation explain how sodium carbonate can be used to soften hard water.(b)Other than softening of hard water give 2 other uses of sodium...(Solved)
a)Using ionic equation explain how sodium carbonate can be used to soften hard water.
b)Other than softening of hard water give 2 other uses of sodium carbonate.
Date posted: September 14, 2017. Answers (1)
- a)Name 2 substances that can be recycled during the manufacture of sodium carbonate.
b)Give 2 uses of calcium chloride.(Solved)
a)Name 2 substances that can be recycled during the manufacture of sodium carbonate.
b)Give 2 uses of calcium chloride.
Date posted: September 14, 2017. Answers (1)
- Predict the pH of the oxide of sulphur in water.(Solved)
Predict the pH of the oxide of sulphur in water.
Date posted: September 14, 2017. Answers (1)
- Write an equation to show the reaction between sulphur and oxygen.(Solved)
Write an equation to show the reaction between sulphur and oxygen.
Date posted: September 14, 2017. Answers (1)
- Explain why sulphur has a higher boiling point compared to that of oxygen. (Solved)
Explain why sulphur has a higher boiling point compared to that of oxygen.
Date posted: September 14, 2017. Answers (1)
- What is the product of sodium carbonate and sulphuric acid?(Solved)
What is the product of sodium carbonate and sulphuric acid?
Date posted: September 14, 2017. Answers (1)
- The standard electrode potentials of a metal G and iron are given below. Fe2+(aq) + 2e---->Fe(s) -0.44VG2+(aq) + 2e---->G(s) ...(Solved)
A piece of iron is coated with metal G. If the coating is scratched, would the iron be protected from rusting? Explain.
Date posted: September 13, 2017. Answers (1)
- Define hydration energy.(b) Given that: the hydration energies of Ca2+ and Cl- are -1891 kJ mol-1 and -384 kJ mol-1 respectively,and that the...(Solved)
(a) Define hydration energy.(1 mark)
(b) Given that: the hydration energies of Ca2+ and Cl- are -1891 kJ mol-1 and -384 kJ mol-1 respectively,and that the lattice energy of calcium chloride is +2237 kJ mol-1.Calculate the molar enthalpy change of solution of calcium chloride.
Date posted: September 13, 2017. Answers (1)
- Study the equation below and answer the questions that follow C6H14 + Cl2--->C6H13Cl + HCl(Solved)
(i) State the condition under which this reaction occurs.(1 mark)
(ii) Give the general name of this type of reaction. (1 mark)
Date posted: September 13, 2017. Answers (1)
- Nitrogen gas can be prepared in the laboratory using a mixture of ammonium chloride solution and sodium nitrite solution.(Solved)
(a) The reaction occurs in two steps. State the two steps in the correct order. (2 marks)
(b) State two applications nitrogen. (1 mark)
Date posted: September 13, 2017. Answers (1)
- A mixture consists of sulphur powder and iron filings.(i) Describe how to obtain sulphur from the mixture using methylbenzene. (Solved)
(i) Describe how to obtain sulphur from the mixture using methylbenzene. (3 marks)
(ii) Is the mixture homogeneous or heterogeneous? Explain. (2 marks)
Date posted: September 13, 2017. Answers (1)
- What is the importance of the shape of a conical flask?(Solved)
What is the importance of the shape of a conical flask?
Date posted: September 13, 2017. Answers (1)
- Using dot and cross to represent electrons draw a diagram to illustrate bonding in the sulphide of a substance with a pH of 2.2(Solved)
Using dot and cross to represent electrons draw a diagram to illustrate bonding in the sulphide of a substance with a pH of 2.2
Date posted: September 10, 2017. Answers (1)
- The graph below shows the mass of a radioactive isotope plotted against time.
(Solved)
The graph below shows the mass of a radioactive isotope plotted against time.
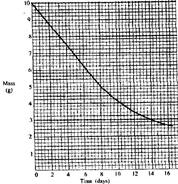
(a) Using the graph, determine the half-life of the isotope.
(b) Calculate the mass of the isotope present after 32 days.
Date posted: June 7, 2017. Answers (1)
- Study the flow chart below and use it to answer the questions that follow. (Solved)
Study the flow chart below and use it to answer the questions that follow.
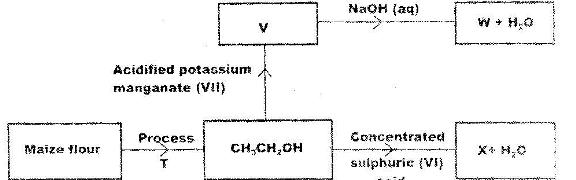
(a) Name process T.
(b) Give the formula of W.
(c) State two uses of X.
Date posted: June 7, 2017. Answers (1)