(a) - Barium Sulphate (BaSO3)
(b) - BaSO3(s) + 2HCI (aq)---> BaCI2(aq) + SO2(aq)
(c) - Changes from orange to green
Kavungya answered the question on June 22, 2019 at 06:42
- The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula...(Solved)
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula of the hydrocarbon
c) To which homologous series does the hydrocarbon drawn in (b) above
belong?
Date posted: June 22, 2019. Answers (1)
- Complete the table below by inserting the missing information in the space
provided.
(Solved)
Complete the table below by inserting the missing information in the space
provided.
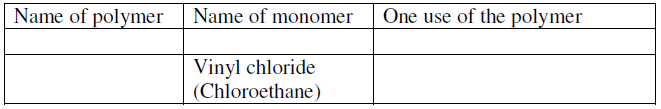
Date posted: June 22, 2019. Answers (1)
- The graph below shows the solubility of sulphur dioxide gas at different
temperatures. Use the following in it to answer the questions that follow.
From the graph...(Solved)
The graph below shows the solubility of sulphur dioxide gas at different
temperatures. Use the following in it to answer the questions that follow.
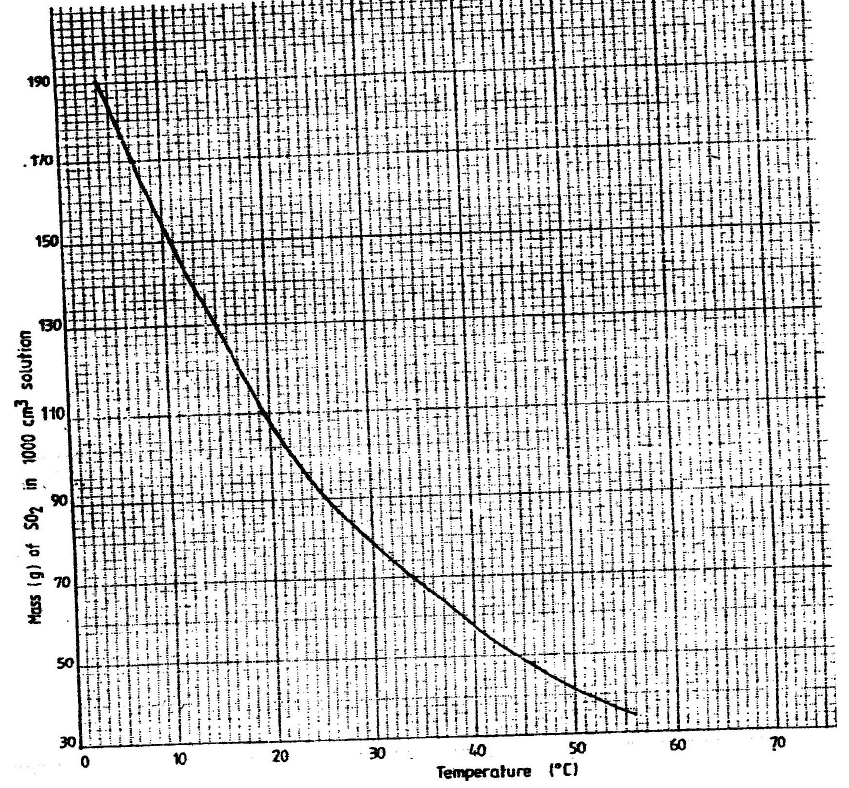
From the graph determine:
I The lowest temperature at which 1,000cm3 of solution would contain
116g of sulphur dioxide.
II The maximum mass of sulphur dioxide that would dissolve in 15 litres of
solution at 100C
Date posted: June 22, 2019. Answers (1)
- Sodium thiosulphate solution reacts with dilute hydrochloric acid according to the
following equation.
S2O32- (aq) + 2H+ (aq) ---> H2O (l) + SO2 + S(s)
In an experiment...(Solved)
Sodium thiosulphate solution reacts with dilute hydrochloric acid according to the
following equation.
S2O32- (aq) + 2H+ (aq) ---> H2O (l) + SO2 + S(s)
In an experiment to study how the rate of reaction varies with concentration,
10cm3 of 0.4M sodium thiosulphate was mixed with 10cm3. Of 2M hydrochloric acid in a
flask. The flask was placed in a white paper marked with across X.The time taken for the
cross X become invisible when viewed from above was noted and recorded in the table
below. The experiment was repeated three times as the temperature using the volumes in
the table and the results recorded as shown in the table below.

a) i) On the grid below, plot a graph of the volume of thiosulphate (Vertical
axis) against time taken for the cross (X) to become invisible)
ii) From the graph determine how long it would take for the cross to become
invisible if the experiment was done.
i) Using 6cm3 of the 0.4M thiosulphate
ii) Using 6cm3 of 0.2M thiosulphate solution
b) i) Using values for experiment I.Calculate
i) Moles of thiosulphate used
ii) Moles of hydrochloric acid used
ii) Explain which of the two reactants in experiment I controlled the rate of
the reaction? Explain
c) Give two precautions which should be taken in experiment I controlled the rate of
the reaction? Explain
Date posted: June 22, 2019. Answers (1)
- Complete the diagram below to show how a and b particles from a radioactive source
can be distinguished from each other. Label your diagram clearly(Solved)
Complete the diagram below to show how a and b particles from a radioactive source
can be distinguished from each other. Label your diagram clearly

Date posted: June 22, 2019. Answers (1)
- Explain why it is not advisable to use aqueous chloride solution as the salt bridge in the
electrochemical cell formed between half cells, Pb2-(aq)/pb(s) E0 =...(Solved)
Explain why it is not advisable to use aqueous chloride solution as the salt bridge in the
electrochemical cell formed between half cells, Pb2-(aq)/pb(s) E0 = 0.13V and
CU2 + (aq) + (aq)/CU2+(aq)/Cu2(s) E0=0.34V
Date posted: June 22, 2019. Answers (1)
- Oxygen reacts with the elements phosphorous, sulphur and chlorine to from oxides oxide
of sulphur and its highest oxidation number. Complete the table for phosphorus and
chlorine....(Solved)
Oxygen reacts with the elements phosphorous, sulphur and chlorine to from oxides oxide
of sulphur and its highest oxidation number. Complete the table for phosphorus and
chlorine. (Atomic numbers: P=15, S= 16 Cl = 17)

Date posted: June 22, 2019. Answers (1)
- On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a
labeled diagram of a set-up that could be used for heating sodium...(Solved)
On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a
labeled diagram of a set-up that could be used for heating sodium nitrate and collecting
the oxygen gas liberated.
Date posted: June 22, 2019. Answers (1)
- Methane reacts with oxygen as shown by the equations I and II below:
Which one of the two reactions represents the complete combustion of methane? Explain(Solved)
Methane reacts with oxygen as shown by the equations I and II below:

Which one of the two reactions represents the complete combustion of methane? Explain
Date posted: June 22, 2019. Answers (1)
- State the number of electrons and neutrons in element H.(Solved)
State the number of electrons and neutrons in element H.
Date posted: June 22, 2019. Answers (1)
- Explain how a sample of CH3CH2CH2OH, could be distinguished from a sample of CH3COOH by means of a chemical reaction(Solved)
Explain how a sample of CH3CH2CH2OH, could be distinguished from a sample of CH3COOH by means of a chemical reaction
Date posted: June 22, 2019. Answers (1)
- The reaction of propane with chlorine gas gave a compound of formula C3H7Cl.
a) What condition is necessary for the above reaction to take place?
b) Draw...(Solved)
The reaction of propane with chlorine gas gave a compound of formula C3H7Cl.
a) What condition is necessary for the above reaction to take place?
b) Draw two structural formulae of the compound C3H7Cl
Date posted: June 22, 2019. Answers (1)
- State why an ammonia molecule (NH3) can combine with H+ to form NH4+
(Atomic numbers: N=7 and H=1)(Solved)
State why an ammonia molecule (NH3) can combine with H+ to form NH4+
(Atomic numbers: N=7 and H=1)
Date posted: June 22, 2019. Answers (1)
- Zinc metal and hydrochloric acid reacts according to the following equation
Zn(s) + 2HCI (aq)---> ZnCI2 (aq) + H2 (g)
1.96 g of zinc were reacted with...(Solved)
Zinc metal and hydrochloric acid reacts according to the following equation
Zn(s) + 2HCI (aq)---> ZnCI2 (aq) + H2 (g)
1.96 g of zinc were reacted with 100cm3of 0.2M hydrochloric acid
(a) Determine the reagent that was in excess
(b) Calculate the total volume of hydrogen gas was liberated S.T.P
(Zn= 65.4 Molar gas volume = 22.4 litres at S.T.P
Date posted: June 22, 2019. Answers (1)
- 233 Pa decays by beta emission. What is the mass number and
91
Atomic number of the element formed?(Solved)
233 Pa decays by beta emission. What is the mass number and
91
Atomic number of the element formed?
Date posted: June 22, 2019. Answers (1)
- 100gm of radioactive 233 Pa was reduced to 12.5g after 81 days.
...(Solved)
100gm of radioactive 233 Pa was reduced to 12.5g after 81 days.
91
Determine the half-life of Pa.
Date posted: June 22, 2019. Answers (1)
- A compound C4H10O_ is oxidized by excess acidified potassium permanganate to..(Solved)
A compound C4H10O_ is oxidized by excess acidified potassium permanganate to
form another compound C4H8O2. The same compound C4H10O reacts with
potassium to produce hydrogen gas.
a) Draw the structural formula and name the compound CaH10O
b) Write an equation for the reaction between potassium and compound
C4H10O.
Date posted: June 22, 2019. Answers (1)
- A compound has an empirical formula, C3H6O and a relative formula mass of 116.
Determine its molecular formula (H + 1.0, C = 12.0, O =...(Solved)
A compound has an empirical formula, C3H6O and a relative formula mass of 116.
Determine its molecular formula (H + 1.0, C = 12.0, O = 165.0)
Date posted: June 22, 2019. Answers (1)
- Duralumin is an alloy of aluminium. What is the advantage of using duralumin
in place of aluminium for manufacture of aeroplane parts.(Solved)
Duralumin is an alloy of aluminium. What is the advantage of using duralumin
in place of aluminium for manufacture of aeroplane parts.
Date posted: June 22, 2019. Answers (1)
- In an experiment, rods of metals P, Q and R were cleaned with sand paper and
placed in a beaker containing water. Another set of rods...(Solved)
In an experiment, rods of metals P, Q and R were cleaned with sand paper and
placed in a beaker containing water. Another set of rods was also cleaned and
placed in a beaker containing dilute acid. After placing the rods in the two liquids
bubbles of gas were seen around some of the rods as shown in the diagrams
below.

a) Why is it necessary to clean the rods with sand paper before dipping them
into the liquids.
b) Arrange the three metals in order of their reactivity starting with the most
reactive.
Date posted: June 22, 2019. Answers (1)