(i) I To produce HCl gas /HCl(g)
II To oxidize HCl (g) to chlorine gas/produce chlorine gas.
(ii) Sodium hypochlorite/ NaOCl / Sodium chlorate
(iii) Kill germs /disinfectant/antiseptic
Kavungya answered the question on June 22, 2019 at 07:13
- A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800...(Solved)
A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800 kg
calculate the mass of ammonia gas used daily.
Date posted: June 22, 2019. Answers (1)
- Calculate the mass of nitrogen dioxide gas that would occupy the same volume as
10g of hydrogen gas at same temperature and pressure.(H = 1.0, N...(Solved)
Calculate the mass of nitrogen dioxide gas that would occupy the same volume as
10g of hydrogen gas at same temperature and pressure.(H = 1.0, N = 14.0, o =
16.0)
Date posted: June 22, 2019. Answers (1)
- When 0.6g of element J were completely burnt in oxygen and all the heat evolved
was used to heat 500cm3 of water, the temperature of the...(Solved)
When 0.6g of element J were completely burnt in oxygen and all the heat evolved
was used to heat 500cm3 of water, the temperature of the water rose from 23oC to
32OC. Calculate the relative atomic mass of element J given that the specific heat
capacity of water = 4.2JK-1
g-1, density of water = 1.0g/cm3 and molar heat is
combustion of J is 380Kjmol-1
Date posted: June 22, 2019. Answers (1)
- Name and draw the structure of the compound formed when methane reacts with
excess chlorine in the presence of U.V light(Solved)
Name and draw the structure of the compound formed when methane reacts with
excess chlorine in the presence of U.V light
Date posted: June 22, 2019. Answers (1)
- 0.63g of lead powder were dissolved in excess nitric acid to form lead nitrate
solution. All the lead nitrate solution was reacted with sodium sulphate solution.
a)Write...(Solved)
0.63g of lead powder were dissolved in excess nitric acid to form lead nitrate
solution. All the lead nitrate solution was reacted with sodium sulphate solution.
a)Write an ionic equation for the reaction between lead nitrate and sodium
sulphate solutions.
b) Determine the mass of the lead salt formed in (a) above.
(Pb = 207, S = 32.0 = 16)
Date posted: June 22, 2019. Answers (1)
- Potassium sulphite solution was prepared and divided into two portions. The first
portion gave a white precipitate when reacted with barium nitrate. On addition of
dilute hydrochloric...(Solved)
Potassium sulphite solution was prepared and divided into two portions. The first
portion gave a white precipitate when reacted with barium nitrate. On addition of
dilute hydrochloric acid the white precipitate disappeared.
a) Write the formula of the compound which formed as the white precipitate.
b) Write the equation for the reaction between dilute hydrochloric acid and
the compound whose formula is written in (a) above.
c) What observation would be made if one drop of potassium dichromate
solution was added to the second portion followed by dilute hydrochloric
acid?
Date posted: June 22, 2019. Answers (1)
- The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula...(Solved)
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula of the hydrocarbon
c) To which homologous series does the hydrocarbon drawn in (b) above
belong?
Date posted: June 22, 2019. Answers (1)
- Complete the table below by inserting the missing information in the space
provided.
(Solved)
Complete the table below by inserting the missing information in the space
provided.
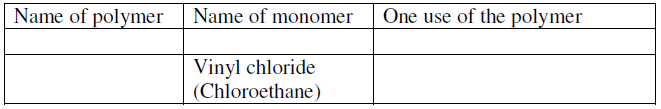
Date posted: June 22, 2019. Answers (1)
- The graph below shows the solubility of sulphur dioxide gas at different
temperatures. Use the following in it to answer the questions that follow.
From the graph...(Solved)
The graph below shows the solubility of sulphur dioxide gas at different
temperatures. Use the following in it to answer the questions that follow.
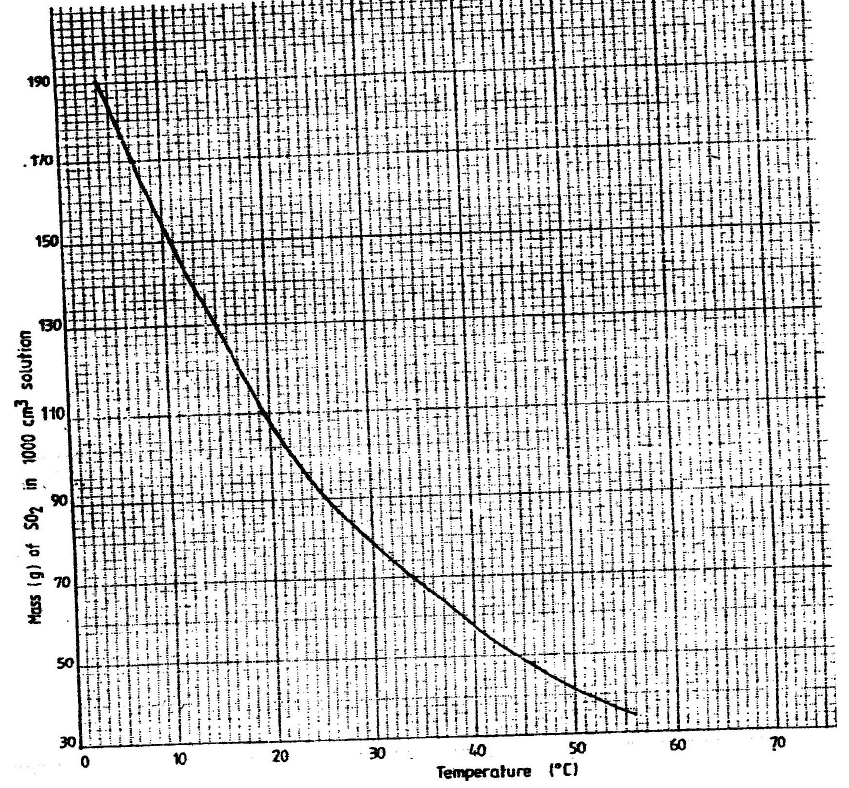
From the graph determine:
I The lowest temperature at which 1,000cm3 of solution would contain
116g of sulphur dioxide.
II The maximum mass of sulphur dioxide that would dissolve in 15 litres of
solution at 100C
Date posted: June 22, 2019. Answers (1)
- Sodium thiosulphate solution reacts with dilute hydrochloric acid according to the
following equation.
S2O32- (aq) + 2H+ (aq) ---> H2O (l) + SO2 + S(s)
In an experiment...(Solved)
Sodium thiosulphate solution reacts with dilute hydrochloric acid according to the
following equation.
S2O32- (aq) + 2H+ (aq) ---> H2O (l) + SO2 + S(s)
In an experiment to study how the rate of reaction varies with concentration,
10cm3 of 0.4M sodium thiosulphate was mixed with 10cm3. Of 2M hydrochloric acid in a
flask. The flask was placed in a white paper marked with across X.The time taken for the
cross X become invisible when viewed from above was noted and recorded in the table
below. The experiment was repeated three times as the temperature using the volumes in
the table and the results recorded as shown in the table below.

a) i) On the grid below, plot a graph of the volume of thiosulphate (Vertical
axis) against time taken for the cross (X) to become invisible)
ii) From the graph determine how long it would take for the cross to become
invisible if the experiment was done.
i) Using 6cm3 of the 0.4M thiosulphate
ii) Using 6cm3 of 0.2M thiosulphate solution
b) i) Using values for experiment I.Calculate
i) Moles of thiosulphate used
ii) Moles of hydrochloric acid used
ii) Explain which of the two reactants in experiment I controlled the rate of
the reaction? Explain
c) Give two precautions which should be taken in experiment I controlled the rate of
the reaction? Explain
Date posted: June 22, 2019. Answers (1)
- Complete the diagram below to show how a and b particles from a radioactive source
can be distinguished from each other. Label your diagram clearly(Solved)
Complete the diagram below to show how a and b particles from a radioactive source
can be distinguished from each other. Label your diagram clearly

Date posted: June 22, 2019. Answers (1)
- Explain why it is not advisable to use aqueous chloride solution as the salt bridge in the
electrochemical cell formed between half cells, Pb2-(aq)/pb(s) E0 =...(Solved)
Explain why it is not advisable to use aqueous chloride solution as the salt bridge in the
electrochemical cell formed between half cells, Pb2-(aq)/pb(s) E0 = 0.13V and
CU2 + (aq) + (aq)/CU2+(aq)/Cu2(s) E0=0.34V
Date posted: June 22, 2019. Answers (1)
- Oxygen reacts with the elements phosphorous, sulphur and chlorine to from oxides oxide
of sulphur and its highest oxidation number. Complete the table for phosphorus and
chlorine....(Solved)
Oxygen reacts with the elements phosphorous, sulphur and chlorine to from oxides oxide
of sulphur and its highest oxidation number. Complete the table for phosphorus and
chlorine. (Atomic numbers: P=15, S= 16 Cl = 17)

Date posted: June 22, 2019. Answers (1)
- On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a
labeled diagram of a set-up that could be used for heating sodium...(Solved)
On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a
labeled diagram of a set-up that could be used for heating sodium nitrate and collecting
the oxygen gas liberated.
Date posted: June 22, 2019. Answers (1)
- Methane reacts with oxygen as shown by the equations I and II below:
Which one of the two reactions represents the complete combustion of methane? Explain(Solved)
Methane reacts with oxygen as shown by the equations I and II below:

Which one of the two reactions represents the complete combustion of methane? Explain
Date posted: June 22, 2019. Answers (1)
- State the number of electrons and neutrons in element H.(Solved)
State the number of electrons and neutrons in element H.
Date posted: June 22, 2019. Answers (1)
- Explain how a sample of CH3CH2CH2OH, could be distinguished from a sample of CH3COOH by means of a chemical reaction(Solved)
Explain how a sample of CH3CH2CH2OH, could be distinguished from a sample of CH3COOH by means of a chemical reaction
Date posted: June 22, 2019. Answers (1)
- The reaction of propane with chlorine gas gave a compound of formula C3H7Cl.
a) What condition is necessary for the above reaction to take place?
b) Draw...(Solved)
The reaction of propane with chlorine gas gave a compound of formula C3H7Cl.
a) What condition is necessary for the above reaction to take place?
b) Draw two structural formulae of the compound C3H7Cl
Date posted: June 22, 2019. Answers (1)
- State why an ammonia molecule (NH3) can combine with H+ to form NH4+
(Atomic numbers: N=7 and H=1)(Solved)
State why an ammonia molecule (NH3) can combine with H+ to form NH4+
(Atomic numbers: N=7 and H=1)
Date posted: June 22, 2019. Answers (1)
- Zinc metal and hydrochloric acid reacts according to the following equation
Zn(s) + 2HCI (aq)---> ZnCI2 (aq) + H2 (g)
1.96 g of zinc were reacted with...(Solved)
Zinc metal and hydrochloric acid reacts according to the following equation
Zn(s) + 2HCI (aq)---> ZnCI2 (aq) + H2 (g)
1.96 g of zinc were reacted with 100cm3of 0.2M hydrochloric acid
(a) Determine the reagent that was in excess
(b) Calculate the total volume of hydrogen gas was liberated S.T.P
(Zn= 65.4 Molar gas volume = 22.4 litres at S.T.P
Date posted: June 22, 2019. Answers (1)