-
A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800...
(Solved)
A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800 kg
calculate the mass of ammonia gas used daily.
Date posted:
June 22, 2019
.
Answers (1)
-
Calculate the mass of nitrogen dioxide gas that would occupy the same volume as
10g of hydrogen gas at same temperature and pressure.(H = 1.0, N...
(Solved)
Calculate the mass of nitrogen dioxide gas that would occupy the same volume as
10g of hydrogen gas at same temperature and pressure.(H = 1.0, N = 14.0, o =
16.0)
Date posted:
June 22, 2019
.
Answers (1)
-
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula...
(Solved)
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula of the hydrocarbon
c) To which homologous series does the hydrocarbon drawn in (b) above
belong?
Date posted:
June 22, 2019
.
Answers (1)
-
Sodium thiosulphate solution reacts with dilute hydrochloric acid according to the
following equation.
S2O32- (aq) + 2H+ (aq) ---> H2O (l) + SO2 + S(s)
In an experiment...
(Solved)
Sodium thiosulphate solution reacts with dilute hydrochloric acid according to the
following equation.
S2O32- (aq) + 2H+ (aq) ---> H2O (l) + SO2 + S(s)
In an experiment to study how the rate of reaction varies with concentration,
10cm3 of 0.4M sodium thiosulphate was mixed with 10cm3. Of 2M hydrochloric acid in a
flask. The flask was placed in a white paper marked with across X.The time taken for the
cross X become invisible when viewed from above was noted and recorded in the table
below. The experiment was repeated three times as the temperature using the volumes in
the table and the results recorded as shown in the table below.

a) i) On the grid below, plot a graph of the volume of thiosulphate (Vertical
axis) against time taken for the cross (X) to become invisible)
ii) From the graph determine how long it would take for the cross to become
invisible if the experiment was done.
i) Using 6cm3 of the 0.4M thiosulphate
ii) Using 6cm3 of 0.2M thiosulphate solution
b) i) Using values for experiment I.Calculate
i) Moles of thiosulphate used
ii) Moles of hydrochloric acid used
ii) Explain which of the two reactants in experiment I controlled the rate of
the reaction? Explain
c) Give two precautions which should be taken in experiment I controlled the rate of
the reaction? Explain
Date posted:
June 22, 2019
.
Answers (1)
-
On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a
labeled diagram of a set-up that could be used for heating sodium...
(Solved)
On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a
labeled diagram of a set-up that could be used for heating sodium nitrate and collecting
the oxygen gas liberated.
Date posted:
June 22, 2019
.
Answers (1)
-
The reaction of propane with chlorine gas gave a compound of formula C3H7Cl.
a) What condition is necessary for the above reaction to take place?
b) Draw...
(Solved)
The reaction of propane with chlorine gas gave a compound of formula C3H7Cl.
a) What condition is necessary for the above reaction to take place?
b) Draw two structural formulae of the compound C3H7Cl
Date posted:
June 22, 2019
.
Answers (1)
-
State why an ammonia molecule (NH3) can combine with H+ to form NH4+
(Atomic numbers: N=7 and H=1)
(Solved)
State why an ammonia molecule (NH3) can combine with H+ to form NH4+
(Atomic numbers: N=7 and H=1)
Date posted:
June 22, 2019
.
Answers (1)
-
Zinc metal and hydrochloric acid reacts according to the following equation
Zn(s) + 2HCI (aq)---> ZnCI2 (aq) + H2 (g)
1.96 g of zinc were reacted with...
(Solved)
Zinc metal and hydrochloric acid reacts according to the following equation
Zn(s) + 2HCI (aq)---> ZnCI2 (aq) + H2 (g)
1.96 g of zinc were reacted with 100cm3of 0.2M hydrochloric acid
(a) Determine the reagent that was in excess
(b) Calculate the total volume of hydrogen gas was liberated S.T.P
(Zn= 65.4 Molar gas volume = 22.4 litres at S.T.P
Date posted:
June 22, 2019
.
Answers (1)
-
Calculate the amount of calcium carbonate that would remain if 15.0g of calcium
carbonate were reacted with 0.2 moles of hydrochloric acid.
The equation for the reaction...
(Solved)
Calculate the amount of calcium carbonate that would remain if 15.0g of calcium
carbonate were reacted with 0.2 moles of hydrochloric acid.
The equation for the reaction is CaCO3 (g) + 2HCl CaCl2 (aq) + CO2 (g) + H2O (g)
(C = 12.0 = 1.60, Ca = 40.0)
Date posted:
June 22, 2019
.
Answers (1)
-
A mass of M grams of radioactive isotopes decay to 5 grams in 100 days. The half-life of the isotope is 25 days. What is the initial...
(Solved)
A mass of M grams of radioactive isotopes decay to 5 grams in 100 days. The half-life of the isotope is 25 days. What is the initial mass of the isotope?
Date posted:
June 13, 2019
.
Answers (1)
-
You are provided with the following reagents; dilute nitric acid, dilute sulphuric acid, and lead (II) oxide. Describe clearly how you would prepare a sample...
(Solved)
You are provided with the following reagents; dilute nitric acid, dilute sulphuric acid, and lead (II) oxide.
Describe clearly how you would prepare a sample of lead (II) sulphate.
Date posted:
June 6, 2019
.
Answers (1)
-
Given a mixture of sodium chloride crystals,ammonium chloride and lead(ii)chloride, describe how you would separate all the four solids using methyl benzene,a source of heat and...
(Solved)
Given a mixture of sodium chloride crystals,ammonium chloride and lead(ii)chloride, describe how you would separate all the four solids using methyl benzene,a source of heat and water.
Date posted:
June 6, 2019
.
Answers (1)
-
Why is potassium nitrate preferred in the laboratory preparation of nitric(V) acid?
(Solved)
Why is potassium nitrate preferred in the laboratory preparation of nitric(V) acid?
Date posted:
June 4, 2019
.
Answers (1)
-
Give the contrasting difference in chemical properties of chlorine and hydrogen?
(Solved)
Give the contrasting difference in chemical properties of chlorine and hydrogen?
Date posted:
May 24, 2019
.
Answers (1)
-
A volume of 15cm3 of ethane gas was exploded with 50cm3 of oxygen. If both volumes were measured at the same temperature and pressure, calculate...
(Solved)
A volume of 15cm3 of ethane gas was exploded with 50cm3 of oxygen. If both volumes were measured at the same temperature and pressure, calculate the volume of resulting gaseous mixture.
Date posted:
May 24, 2019
.
Answers (1)
-
An element Q has a relative atomic mass of 88. When a current of 0.5A was passed through the fused chloride of Q for 32
minutes...
(Solved)
An element Q has a relative atomic mass of 88. When a current of 0.5A was passed through the fused chloride of Q for 32 minutes and 10 seconds, 0.44g of Q were deposited at cathode. Determine the charge on the ion of Q. (1 Faraday = 9650
Date posted:
May 24, 2019
.
Answers (1)
-
The electronic structures for elements represented by letters A, B, C and D are
A - 2.8.6 B - 2.8.2 C - 2.8.1 D - 2.8.8
a)...
(Solved)
The electronic structures for elements represented by letters A, B, C and D are
A - 2.8.6 B - 2.8.2 C - 2.8.1 D - 2.8.8
a) Select the element which forms :
i) a double charged cation
ii) a soluble carbonate
b) Which element has the shortest atomic radius ?
Date posted:
May 24, 2019
.
Answers (1)
-
Metal S removes oxygen combined with P. Q reacts with an oxide of R and not with an oxide of P. P reacts with cold...
(Solved)
Metal S removes oxygen combined with P. Q reacts with an oxide of R and not with an oxide of P. P reacts with cold water
but Q does not.
a) Which is the most reactive metal ?
b) Which is the least reactive metal ?
c) Arrange the metals in order of reactivity starting with most reactive to the least reactive.
Date posted:
May 24, 2019
.
Answers (1)
-
Below are PH values of some solutions.
i) Which solution is likely to be
I. acidic rain
II. Potassium hydroxide
ii) A basic substance V...
(Solved)
Below are PH values of some solutions.

i) Which solution is likely to be
I. acidic rain
II. Potassium hydroxide
ii) A basic substance V reacted with both solutions Y and X. What is the nature of V.
iii) Identify two substances that show these characteristics in question (ii) above.
Date posted:
May 24, 2019
.
Answers (1)
-
The diagram below is a section of a model of the structure of element T.
a) State the type of bonding that exist in T.
b) In...
(Solved)
The diagram below is a section of a model of the structure of element T.
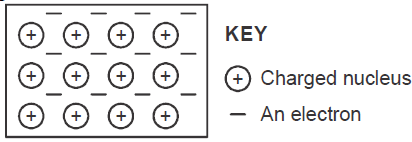
a) State the type of bonding that exist in T.
b) In which group of the periodic table does element T belong ? Give a reason.
Date posted:
May 24, 2019
.
Answers (1)