- The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted
into two other hydrocarbons as shown by the equation below
(i) Name and draw...(Solved)
The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted
into two other hydrocarbons as shown by the equation below
(i) Name and draw the possible structural formula of X
Name
Structural formula
(ii) State and explain the observation that would be made if a few drops of bromide
water were added to a sample of X.
(iii) Write an equation for the complete combustion of C3H8
Date posted: June 22, 2019. Answers (1)
- The fermentation of glucose produces ethanol as shown in the equation below
C2H12O6(aq) ---> 2CH3CH2OH(aq) + 2CO2(g)
(i) State how the concentration of ethanol produced could be...(Solved)
The fermentation of glucose produces ethanol as shown in the equation below
C2H12O6(aq) ---> 2CH3CH2OH(aq) + 2CO2(g)
(i) State how the concentration of ethanol produced could be increased
(ii) State and explain the observation that would be made when a piece of sodium
metal is added to a sample of ethanol contained in a beaker
(iii) Give two commercial uses of ethanol other in the manufacture of alcoholic
drinks
Date posted: June 22, 2019. Answers (1)
- The following equations represents two different types of reactions
State the type of reaction represented by:
(i)
(ii)(Solved)
The following equations represents two different types of reactions
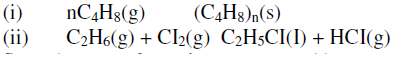
State the type of reaction represented by:
(i)
(ii)
Date posted: June 22, 2019. Answers (1)
- Corrosive is a destructive process in which iron which is converted into hydrated iron
(III) oxide
State:
(i) two conditions necessary for rusting to occur
(ii) One method used...(Solved)
Corrosive is a destructive process in which iron which is converted into hydrated iron
(III) oxide
State:
(i) two conditions necessary for rusting to occur
(ii) One method used to protect iron from rusting
Date posted: June 22, 2019. Answers (1)
- An ore is suspected to contain mainly iron. Describe a method that
can be used to confirm the presence of iron in the ore(Solved)
An ore is suspected to contain mainly iron. Describe a method that
can be used to confirm the presence of iron in the ore
Date posted: June 22, 2019. Answers (1)
- What is the oxidation number of chlorine in CO4?(Solved)
What is the oxidation number of chlorine in CO4?
Date posted: June 22, 2019. Answers (1)
- A sealed glass tube containing air at s.t.p was immersed in water at 1000c. Assuming
that there was no increase in the volume of the glass...(Solved)
A sealed glass tube containing air at s.t.p was immersed in water at 1000c. Assuming
that there was no increase in the volume of the glass tube due to the expansion of the
glass, calculate the pressure of the inside tube. (standard pressure = 760mmHg,)
Date posted: June 22, 2019. Answers (1)
- Draw the structural formula of:
(a) Ethanol
(b) Propanoic
(c) Give the name of the organic compound formed when ethanol and propanoic
acid react in the presence of concentrated...(Solved)
Draw the structural formula of:
(a) Ethanol
(b) Propanoic
(c) Give the name of the organic compound formed when ethanol and propanoic
acid react in the presence of concentrated sulphuric acid.
Date posted: June 22, 2019. Answers (1)
- State and explain the observation that would be made if a few pellets of potassium
hydroxide are added to the equilibrium mixture.(Solved)
State and explain the observation that would be made if a few pellets of potassium
hydroxide are added to the equilibrium mixture.
Date posted: June 22, 2019. Answers (1)
- On complete combustion of a sample of hydrocarbon, 3.52 gm of carbon dioxide and
1.44g of water were formed. Determine the molecular formula of the hydrocarbon.
(Relative...(Solved)
On complete combustion of a sample of hydrocarbon, 3.52 gm of carbon dioxide and
1.44g of water were formed. Determine the molecular formula of the hydrocarbon.
(Relative molecular masses of hydrocarbon =56, carbon dioxide 44, water = 18 and
relative atomic masses H = 1.0 and c=12.0)
Date posted: June 22, 2019. Answers (1)
- An isotope of Uranium 234U, decays by emission of an alpha particle to thorium
...(Solved)
An isotope of Uranium 234U, decays by emission of an alpha particle to thorium
92
a) Write the equation for the nuclear reaction undergone by isotope.
b) Explain why it is not safe to store radioactive substances in containers made
from aluminium sheets.
Date posted: June 22, 2019. Answers (1)
- 1.9 g of magnesium chloride was dissolved in distilled water. Silver
nitrate solution was added until in excess. Calculate the mass of silver
nitrate that was used...(Solved)
1.9 g of magnesium chloride was dissolved in distilled water. Silver
nitrate solution was added until in excess. Calculate the mass of silver
nitrate that was used for the complete reaction. Relative molecular mass of
magnesium chloride = 95, N = 14.0, O = 16.0, Ag = 108.0
Date posted: June 22, 2019. Answers (1)
- Chloride can be prepared by using the following three agents; solid
sodium chloride, concentrated sulphuric acid and potassium permanganate
(i) What is the role of each of...(Solved)
Chloride can be prepared by using the following three agents; solid
sodium chloride, concentrated sulphuric acid and potassium permanganate
(i) What is the role of each of the following in the reaction?
I Concentrated sulphuric acid
II potassium permanganate
(ii) Name the bleaching agent formed when chlorine gas is passed through
cold dilute sodium hydroxide solution
(iii) Name one other use of the compound formed in (ii) above other than
bleaching
Date posted: June 22, 2019. Answers (1)
- A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800...(Solved)
A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800 kg
calculate the mass of ammonia gas used daily.
Date posted: June 22, 2019. Answers (1)
- Calculate the mass of nitrogen dioxide gas that would occupy the same volume as
10g of hydrogen gas at same temperature and pressure.(H = 1.0, N...(Solved)
Calculate the mass of nitrogen dioxide gas that would occupy the same volume as
10g of hydrogen gas at same temperature and pressure.(H = 1.0, N = 14.0, o =
16.0)
Date posted: June 22, 2019. Answers (1)
- When 0.6g of element J were completely burnt in oxygen and all the heat evolved
was used to heat 500cm3 of water, the temperature of the...(Solved)
When 0.6g of element J were completely burnt in oxygen and all the heat evolved
was used to heat 500cm3 of water, the temperature of the water rose from 23oC to
32OC. Calculate the relative atomic mass of element J given that the specific heat
capacity of water = 4.2JK-1
g-1, density of water = 1.0g/cm3 and molar heat is
combustion of J is 380Kjmol-1
Date posted: June 22, 2019. Answers (1)
- Name and draw the structure of the compound formed when methane reacts with
excess chlorine in the presence of U.V light(Solved)
Name and draw the structure of the compound formed when methane reacts with
excess chlorine in the presence of U.V light
Date posted: June 22, 2019. Answers (1)
- 0.63g of lead powder were dissolved in excess nitric acid to form lead nitrate
solution. All the lead nitrate solution was reacted with sodium sulphate solution.
a)Write...(Solved)
0.63g of lead powder were dissolved in excess nitric acid to form lead nitrate
solution. All the lead nitrate solution was reacted with sodium sulphate solution.
a)Write an ionic equation for the reaction between lead nitrate and sodium
sulphate solutions.
b) Determine the mass of the lead salt formed in (a) above.
(Pb = 207, S = 32.0 = 16)
Date posted: June 22, 2019. Answers (1)
- Potassium sulphite solution was prepared and divided into two portions. The first
portion gave a white precipitate when reacted with barium nitrate. On addition of
dilute hydrochloric...(Solved)
Potassium sulphite solution was prepared and divided into two portions. The first
portion gave a white precipitate when reacted with barium nitrate. On addition of
dilute hydrochloric acid the white precipitate disappeared.
a) Write the formula of the compound which formed as the white precipitate.
b) Write the equation for the reaction between dilute hydrochloric acid and
the compound whose formula is written in (a) above.
c) What observation would be made if one drop of potassium dichromate
solution was added to the second portion followed by dilute hydrochloric
acid?
Date posted: June 22, 2019. Answers (1)
- The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula...(Solved)
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has
a relative molecular mass of 54..(H = 1.0, C = 12.0).
a) C2H3
b) Draw the structural formula of the hydrocarbon
c) To which homologous series does the hydrocarbon drawn in (b) above
belong?
Date posted: June 22, 2019. Answers (1)