I – Manganese (iv) Oxide is a catalyst and increases the rate of decomposition
of the hydrogen peroxide.
Kavungya answered the question on June 24, 2019 at 05:47
- A given volume of ozone, (O3) diffused from a certain apparatus in 96 seconds.
Calculate the time taken by an equal volume of carbon dioxide (CO2)...(Solved)
A given volume of ozone, (O3) diffused from a certain apparatus in 96 seconds.
Calculate the time taken by an equal volume of carbon dioxide (CO2) to diffuse under
the same conditions (O = 16.0, C = 12.0)
Date posted: June 24, 2019. Answers (1)
- 20.0cm3 of a solution containing 4 gm per litre of sodium hydroxide was neutralized
by 8.0cm3 of dilute sulphuric acid. Calculate the concentration of sulphuric acid...(Solved)
20.0cm3 of a solution containing 4 gm per litre of sodium hydroxide was neutralized
by 8.0cm3 of dilute sulphuric acid. Calculate the concentration of sulphuric acid in
moles per litre (Na = 23.0, O = 16.0, H = 1.0)
Date posted: June 24, 2019. Answers (1)
- Use the scheme below to answer the questions that follow
(a) Identify the solid
H
J
(b) State one commercial use of solid J(Solved)
Use the scheme below to answer the questions that follow
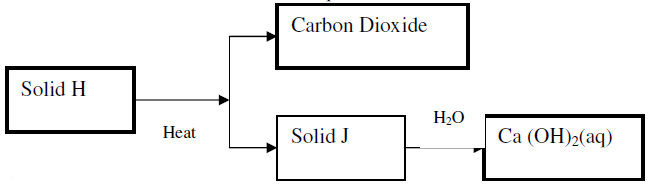
(a) Identify the solid
H
J
(b) State one commercial use of solid J
Date posted: June 24, 2019. Answers (1)
- A hydrocarbon P was found to decolourise bromine water. On complete combustion of 2
moles of P, 6 moles of carbon dioxide and 6 moles of...(Solved)
A hydrocarbon P was found to decolourise bromine water. On complete combustion of 2
moles of P, 6 moles of carbon dioxide and 6 moles of water were formed
(a) Write the structural formula of P
(b) Give the name of P
(c) Name one industrial source of P
Date posted: June 24, 2019. Answers (1)
- The information below relates to elements S,T,U and X. ( the letters do not represents the
actual symbols of the elements.
(i) T displaces X from an...(Solved)
The information below relates to elements S,T,U and X. ( the letters do not represents the
actual symbols of the elements.
(i) T displaces X from an aqueous solution containing ions of X
(ii) Hydrogen gas reduces heated oxide of S but does not reduce the heated oxide of X
(iii) U liberates hydrogen gas from cold water but T does not
(a) Write an equation for the reaction between T and the ions of X.
Both T and X are in group II of the periodic Table)
(b) Arrange the elements in order of their increasing reactivity
Date posted: June 24, 2019. Answers (1)
- When a current of 1.5 amperes was passed though a cell containing m3+ ions of a metal
M for 15 minutes, the mass of the cathode...(Solved)
When a current of 1.5 amperes was passed though a cell containing m3+ ions of a metal
M for 15 minutes, the mass of the cathode increased by 0.26g.
(1 Faraday = 96500 coulombs)
(a) Calculate the quantity of electricity used
(b) Determine the relative atomic mass of metal M
Date posted: June 22, 2019. Answers (1)
- The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted
into two other hydrocarbons as shown by the equation below
(i) Name and draw...(Solved)
The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted
into two other hydrocarbons as shown by the equation below
(i) Name and draw the possible structural formula of X
Name
Structural formula
(ii) State and explain the observation that would be made if a few drops of bromide
water were added to a sample of X.
(iii) Write an equation for the complete combustion of C3H8
Date posted: June 22, 2019. Answers (1)
- The fermentation of glucose produces ethanol as shown in the equation below
C2H12O6(aq) ---> 2CH3CH2OH(aq) + 2CO2(g)
(i) State how the concentration of ethanol produced could be...(Solved)
The fermentation of glucose produces ethanol as shown in the equation below
C2H12O6(aq) ---> 2CH3CH2OH(aq) + 2CO2(g)
(i) State how the concentration of ethanol produced could be increased
(ii) State and explain the observation that would be made when a piece of sodium
metal is added to a sample of ethanol contained in a beaker
(iii) Give two commercial uses of ethanol other in the manufacture of alcoholic
drinks
Date posted: June 22, 2019. Answers (1)
- The following equations represents two different types of reactions
State the type of reaction represented by:
(i)
(ii)(Solved)
The following equations represents two different types of reactions
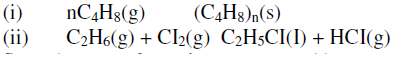
State the type of reaction represented by:
(i)
(ii)
Date posted: June 22, 2019. Answers (1)
- Corrosive is a destructive process in which iron which is converted into hydrated iron
(III) oxide
State:
(i) two conditions necessary for rusting to occur
(ii) One method used...(Solved)
Corrosive is a destructive process in which iron which is converted into hydrated iron
(III) oxide
State:
(i) two conditions necessary for rusting to occur
(ii) One method used to protect iron from rusting
Date posted: June 22, 2019. Answers (1)
- An ore is suspected to contain mainly iron. Describe a method that
can be used to confirm the presence of iron in the ore(Solved)
An ore is suspected to contain mainly iron. Describe a method that
can be used to confirm the presence of iron in the ore
Date posted: June 22, 2019. Answers (1)
- What is the oxidation number of chlorine in CO4?(Solved)
What is the oxidation number of chlorine in CO4?
Date posted: June 22, 2019. Answers (1)
- A sealed glass tube containing air at s.t.p was immersed in water at 1000c. Assuming
that there was no increase in the volume of the glass...(Solved)
A sealed glass tube containing air at s.t.p was immersed in water at 1000c. Assuming
that there was no increase in the volume of the glass tube due to the expansion of the
glass, calculate the pressure of the inside tube. (standard pressure = 760mmHg,)
Date posted: June 22, 2019. Answers (1)
- Draw the structural formula of:
(a) Ethanol
(b) Propanoic
(c) Give the name of the organic compound formed when ethanol and propanoic
acid react in the presence of concentrated...(Solved)
Draw the structural formula of:
(a) Ethanol
(b) Propanoic
(c) Give the name of the organic compound formed when ethanol and propanoic
acid react in the presence of concentrated sulphuric acid.
Date posted: June 22, 2019. Answers (1)
- State and explain the observation that would be made if a few pellets of potassium
hydroxide are added to the equilibrium mixture.(Solved)
State and explain the observation that would be made if a few pellets of potassium
hydroxide are added to the equilibrium mixture.
Date posted: June 22, 2019. Answers (1)
- On complete combustion of a sample of hydrocarbon, 3.52 gm of carbon dioxide and
1.44g of water were formed. Determine the molecular formula of the hydrocarbon.
(Relative...(Solved)
On complete combustion of a sample of hydrocarbon, 3.52 gm of carbon dioxide and
1.44g of water were formed. Determine the molecular formula of the hydrocarbon.
(Relative molecular masses of hydrocarbon =56, carbon dioxide 44, water = 18 and
relative atomic masses H = 1.0 and c=12.0)
Date posted: June 22, 2019. Answers (1)
- An isotope of Uranium 234U, decays by emission of an alpha particle to thorium
...(Solved)
An isotope of Uranium 234U, decays by emission of an alpha particle to thorium
92
a) Write the equation for the nuclear reaction undergone by isotope.
b) Explain why it is not safe to store radioactive substances in containers made
from aluminium sheets.
Date posted: June 22, 2019. Answers (1)
- 1.9 g of magnesium chloride was dissolved in distilled water. Silver
nitrate solution was added until in excess. Calculate the mass of silver
nitrate that was used...(Solved)
1.9 g of magnesium chloride was dissolved in distilled water. Silver
nitrate solution was added until in excess. Calculate the mass of silver
nitrate that was used for the complete reaction. Relative molecular mass of
magnesium chloride = 95, N = 14.0, O = 16.0, Ag = 108.0
Date posted: June 22, 2019. Answers (1)
- Chloride can be prepared by using the following three agents; solid
sodium chloride, concentrated sulphuric acid and potassium permanganate
(i) What is the role of each of...(Solved)
Chloride can be prepared by using the following three agents; solid
sodium chloride, concentrated sulphuric acid and potassium permanganate
(i) What is the role of each of the following in the reaction?
I Concentrated sulphuric acid
II potassium permanganate
(ii) Name the bleaching agent formed when chlorine gas is passed through
cold dilute sodium hydroxide solution
(iii) Name one other use of the compound formed in (ii) above other than
bleaching
Date posted: June 22, 2019. Answers (1)
- A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800...(Solved)
A factory uses nitric acid and ammonia gas as the only reactants for the
preparation of fertilizer. If the daily production of the fertilizer is 4800 kg
calculate the mass of ammonia gas used daily.
Date posted: June 22, 2019. Answers (1)