Distillation/ Fractional distillation
Kavungya answered the question on June 24, 2019 at 07:14
- Name one suitable drying agent for ammonia gas.(Solved)
Name one suitable drying agent for ammonia gas.
Date posted: June 24, 2019. Answers (1)
- When excess lead nitrate solution was added to a solution containing sodium chloride,
the precipitate formed was found to weigh 5.56 g. Determine the amount of...(Solved)
When excess lead nitrate solution was added to a solution containing sodium chloride,
the precipitate formed was found to weigh 5.56 g. Determine the amount of sodium
chloride in the solution ( Pb = 207, Cl = 35.5 Na = 23)
PbXO3(aq) + 2NaCl (aq) → PbCI2 + NaNO3 (aq) (l)
Date posted: June 24, 2019. Answers (1)
- When carbon dioxide gas was passed through aqueous calcium hydroxide a white
suspension was formed
(a) Write an equation for the reaction that took place
(b) State and...(Solved)
When carbon dioxide gas was passed through aqueous calcium hydroxide a white
suspension was formed
(a) Write an equation for the reaction that took place
(b) State and explain the changes that would occur when carbon dioxide gas is bubbled
through the white suspension
Date posted: June 24, 2019. Answers (1)
- The following two tests were carried out on chlorine water contained in two test tubes
a) A piece of blue flower was dropped into the first...(Solved)
The following two tests were carried out on chlorine water contained in two test tubes
a) A piece of blue flower was dropped into the first – tube. Explain why the flower
was bleached
(b) The second test- tube was corked and exposed to sunlight after a few days, it was
found to contain a gas that rekindled a glowing splint. Write an equation for the
reaction which produced the gas
Date posted: June 24, 2019. Answers (1)
- In an experiment to study the rate for reaction between duralumin (an alloy of aluminium,
magnesium and copper) and hydrochloric acid, 0.5g of the alloy were...(Solved)
In an experiment to study the rate for reaction between duralumin (an alloy of aluminium,
magnesium and copper) and hydrochloric acid, 0.5g of the alloy were reacted with excess
4M hydrochloric acid. The data in the table below was recoded.
Use it to answer the questions that follow.

a) i) On the grid provided, plot a graph of total volume of gas produced (vertical axis)
again time.
ii) From the graph, determine the volume of gas produced at the end of 2 ½ minutes.
b) Determine the rate of reaction between the 3rd and 4th minute.
c) Give a reason why some solid remained at the end of the experiment
d) Given that 2.5cm3 of the total volume of the gas was from the reaction between
magnesium and aqueous hydrochloric acid, calculate the percentage mass of aluminium
present in 0.5g of the alloy.
(Al = 27.0 and Molar gas volume = 24,000cm3 at 298k)
e) State two properties of duralumin that make it more suitable than aluminium in aeroplane
construction.
Date posted: June 24, 2019. Answers (1)
- Explain why hydrogen forms compounds in which its oxidation state is either + 1 or -1
(Atomic number of hydrogen is 1)(Solved)
Explain why hydrogen forms compounds in which its oxidation state is either + 1 or -1
(Atomic number of hydrogen is 1)
Date posted: June 24, 2019. Answers (1)
- In the presence of U.V light, ethane gas undergoes substitution reaction with chlorine.
(a) What is meant by the term?
Substitution reaction:
(b) Give the structural formula and...(Solved)
In the presence of U.V light, ethane gas undergoes substitution reaction with chlorine.
(a) What is meant by the term?
Substitution reaction:
(b) Give the structural formula and the name of the organic product formed when
equal volumes of ethane and chlorine react together.
Date posted: June 24, 2019. Answers (1)
- When a sample of concentrated sulphuric acid was left in an open beaker in a room for
two days, the volume was found to have increased...(Solved)
When a sample of concentrated sulphuric acid was left in an open beaker in a room for
two days, the volume was found to have increased slightly
a) What property of concentrated sulphuric acid was left in an open beaker in a room
for two days, the volume was found to have increased slightly.
b) State one use of concentrated sulphuric acid that depends on the property named
above.
Date posted: June 24, 2019. Answers (1)
- A weighed sample of crystalline sodium carbonate (Na2CO3. H2O) was heated in a
crucible until there was no further change in mass.
Calculate the number of moles...(Solved)
A weighed sample of crystalline sodium carbonate (Na2CO3. H2O) was heated in a
crucible until there was no further change in mass.
Calculate the number of moles (n) of the water of crystallization
Date posted: June 24, 2019. Answers (1)
- Sample solutions of salt were labeled as I,II, III and IV. The actual solutions, not in that
order are lead nitrate, zinc sulphate potassium chloride and...(Solved)
Sample solutions of salt were labeled as I,II, III and IV. The actual solutions, not in that
order are lead nitrate, zinc sulphate potassium chloride and calcium chloride.
a) When aqueous sodium carbonate was added to each sample separately, a white
precipitate was formed in I, III and IV only. Identify solution II.
b) When excess sodium hydroxide was added to each sample separately, a white
precipitate was formed in solutions III and I only.
Identify solution I
Date posted: June 24, 2019. Answers (1)
- At 298K and 1 atmosphere, graphite changes into diamond according to the equation:
C (graphite) ? C(diamond); ?=2.9 kJmo-1
In the space provided, sketch a simple energy...(Solved)
At 298K and 1 atmosphere, graphite changes into diamond according to the equation:
C (graphite) → C(diamond); △=2.9 kJmo-1
In the space provided, sketch a simple energy level diagram for the above change.
Date posted: June 24, 2019. Answers (1)
- In an experiment, 0.8gm of magnesium of powder were reacted with excess dilute
sulphuric acid at 250C . The time for the reaction to come to...(Solved)
In an experiment, 0.8gm of magnesium of powder were reacted with excess dilute
sulphuric acid at 250C . The time for the reaction to come to completion was recorded.
The experiment was repeated at 400C. In which experiment was the time taken shorter?
Explain your answer.
Date posted: June 24, 2019. Answers (1)
- It was confirmed that magnesium sulphate was present in the tap water
(i) What type of hardness was present in the water?
(ii) Explain how the hardness...(Solved)
It was confirmed that magnesium sulphate was present in the tap water
(i) What type of hardness was present in the water?
(ii) Explain how the hardness can be removed
Date posted: June 24, 2019. Answers (1)
- (i) In the space provided sketch a labeled diagram to show how hydrogen
chloride gas can be prepared and collected in the laboratory using sodium
Chloride and...(Solved)
(i) In the space provided sketch a labeled diagram to show how hydrogen
chloride gas can be prepared and collected in the laboratory using sodium
Chloride and concentrated sulphuric acid ( the gas need not be dry)
(ii) Write an equation for the reaction that takes place
(iii) Name one drying agent for hydrogen chloride
(vi) State and explain the observation that would be made when hydrogen
chloride gas is bubbled through a solution of lead (II) nitrate
(v) Concentrated hydrochloric acid is used for removing oxide from metal surfaces (
picking). Explain why concentration nitric acid cannot be used for the purpose.
Date posted: June 24, 2019. Answers (1)
- The curves below were obtained when two equal volumes of hydrogen peroxide of the
same concentration were allowed to decompose separately. In one case, manganese (IV)
oxide...(Solved)
The curves below were obtained when two equal volumes of hydrogen peroxide of the
same concentration were allowed to decompose separately. In one case, manganese (IV)
oxide was added to the hydrogen peroxide
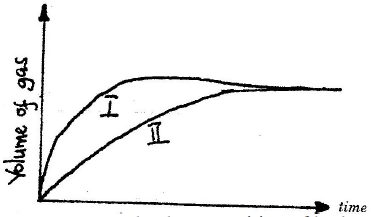
Which curve represents the decomposition of hydrogen peroxide with manganese (IV)
oxide? Explain
Date posted: June 24, 2019. Answers (1)
- A given volume of ozone, (O3) diffused from a certain apparatus in 96 seconds.
Calculate the time taken by an equal volume of carbon dioxide (CO2)...(Solved)
A given volume of ozone, (O3) diffused from a certain apparatus in 96 seconds.
Calculate the time taken by an equal volume of carbon dioxide (CO2) to diffuse under
the same conditions (O = 16.0, C = 12.0)
Date posted: June 24, 2019. Answers (1)
- 20.0cm3 of a solution containing 4 gm per litre of sodium hydroxide was neutralized
by 8.0cm3 of dilute sulphuric acid. Calculate the concentration of sulphuric acid...(Solved)
20.0cm3 of a solution containing 4 gm per litre of sodium hydroxide was neutralized
by 8.0cm3 of dilute sulphuric acid. Calculate the concentration of sulphuric acid in
moles per litre (Na = 23.0, O = 16.0, H = 1.0)
Date posted: June 24, 2019. Answers (1)
- Use the scheme below to answer the questions that follow
(a) Identify the solid
H
J
(b) State one commercial use of solid J(Solved)
Use the scheme below to answer the questions that follow
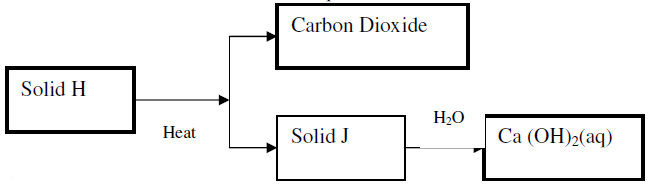
(a) Identify the solid
H
J
(b) State one commercial use of solid J
Date posted: June 24, 2019. Answers (1)
- A hydrocarbon P was found to decolourise bromine water. On complete combustion of 2
moles of P, 6 moles of carbon dioxide and 6 moles of...(Solved)
A hydrocarbon P was found to decolourise bromine water. On complete combustion of 2
moles of P, 6 moles of carbon dioxide and 6 moles of water were formed
(a) Write the structural formula of P
(b) Give the name of P
(c) Name one industrial source of P
Date posted: June 24, 2019. Answers (1)
- The information below relates to elements S,T,U and X. ( the letters do not represents the
actual symbols of the elements.
(i) T displaces X from an...(Solved)
The information below relates to elements S,T,U and X. ( the letters do not represents the
actual symbols of the elements.
(i) T displaces X from an aqueous solution containing ions of X
(ii) Hydrogen gas reduces heated oxide of S but does not reduce the heated oxide of X
(iii) U liberates hydrogen gas from cold water but T does not
(a) Write an equation for the reaction between T and the ions of X.
Both T and X are in group II of the periodic Table)
(b) Arrange the elements in order of their increasing reactivity
Date posted: June 24, 2019. Answers (1)