Volume of the drop =0.24mm³
Area of the patch =4.2 x 10^4mm^2
v= Ah
h = V/A =0.24/(4.2×?10?^4 )
h=5.714×?10?^(-6)mm
Thickness= 5.714 x 10^-9 m.
Githiari answered the question on September 16, 2017 at 07:27
-
Explain why the smell of a rotting body is very strong when the sun is hot as compared to early in the morning.
(Solved)
Explain why the smell of a rotting body is very strong when the sun is hot as compared to early in the morning.
Date posted:
September 16, 2017
.
Answers (1)
-
An uncharged metal rod is brought close but not touching the cap of a charged electroscope causes a decrease in the divergence of the leaf.
(Solved)
An uncharged metal rod is brought close but not touching the cap of a charged electroscope causes a decrease in the divergence of the leaf. Explain.
Date posted:
September 15, 2017
.
Answers (1)
-
Why are the caps of the cell of a lead acid accumulator opened when charging the battery?
(Solved)
Why are the caps of the cell of a lead acid accumulator opened when charging the battery?
Date posted:
September 12, 2017
.
Answers (1)
-
Why do bus body builders build luggage compartments under the seats rather than on roof rack?
(Solved)
Why do bus body builders build luggage compartments under the seats rather than on roof rack?
Date posted:
September 9, 2017
.
Answers (1)
-
I bought some red pens at sh 6 each and some blue pens at sh 4 each. If I bought 38 pens in total and paid sh 192 for them, find the number of red and blue pens I bought.
(Solved)
I bought some red pens at sh 6 each and some blue pens at sh 4 each. If I bought 38 pens in total and paid sh 192 for them, find the number of red and blue pens I bought.
Date posted:
June 8, 2017
.
Answers (1)
-
Figure 4 shows the cross-section of a diffusion cloud chamber used to detect radiation from radioctive sources.
(Solved)
Figure 4 shows the cross-section of a diffusion cloud chamber used to detect radiation from radioctive sources.
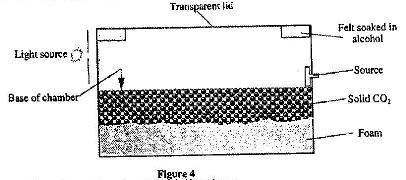
(i) State one function of each of the following:
Alcohol
Solid CO2
(ii) When radiation from the source enters the chamber, some white traces are observed. Explain how these traces are formed and state how the radiation is identified.
(iii) A leaf electroscope can also be used as a detector of radiation. State two advantages of the diffusion cloud chamber over the leaf electroscope as a detector.
Date posted:
June 6, 2017
.
Answers (1)
-
Figure 4 shows a Geiger Muller (G.M,) tube.
(Solved)
Figure 4 shows a Geiger Muller (G.M,) tube.
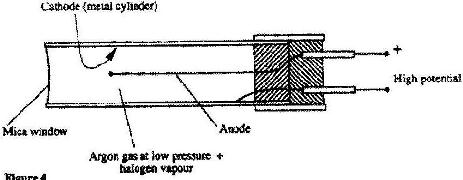
(i) Give the reason why the mica window is made thin
(ii) Explain how the radiation entering the tube through the window is detected by the tube.
(iii) What is the purpose of the halogen vapour?
Date posted:
June 6, 2017
.
Answers (1)
-
Figure 8 shows ultra-violet light striking a polished zinc plate placed on a negatively charged gold-leaf electroscope.
(Solved)
Figure 8 shows ultra-violet light striking a polished zinc plate placed on a negatively charged gold-leaf electroscope.

Explain the following observations.
(i) The leaf of the electroscope falls.
(ii) When the same experiment was repeated with a positively charged electroscope the leaf did not fall
Date posted:
June 6, 2017
.
Answers (1)
-
Explain how land and sea breeze occurs
(Solved)
Explain how land and sea breeze occurs.
Date posted:
June 6, 2017
.
Answers (1)
-
Figure 18 shows the parts of an x-ray tube.
(Solved)
Figure 18 shows the parts of an x-ray tube.

(a) Explain why:
(i) A potential difference is applied to the filament.
(ii) A high potential difference is applied between a cathode and the anode.
(iii) Most of the tube is surrounded by lead.
(b) State how the resulting x -rays are affected by increasing the potential difference between the anode and the cathode.
Date posted:
June 6, 2017
.
Answers (1)
-
Figure 13 shows the features of an X-ray tube.
(Solved)
Figure 13 shows the features of an X-ray tube.

(i) Name the parts labeled A and B.
(ii) Explain how a change in the potential across PQ changes the intensity of the X -rays produced in the tube.
(iii) During the operation of the tube, the target becomes very hot. Explain how this heat is caused.
(iv) What property of lead makes it suitable for use as shielding material?
Date posted:
June 6, 2017
.
Answers (1)
-
Figure 10 shows the main features of a cathode ray oscilloscope (CRO).
(Solved)
Figure 10 shows the main features of a cathode ray oscilloscope (CRO).

(i) Name the part labeled M and N.
(ii) Explain how electrons are produced in the tube.
(iii) State why the tube is highly evacuated.
Date posted:
June 6, 2017
.
Answers (1)
-
Figure 3 shows the main features of a cathode ray tube (CRT) of a cathode ray oscilloscope (CRO).
(Solved)
Figure 3 shows the main features of a cathode ray tube (CRT) of a cathode ray oscilloscope (CRO).

(i) Describe how the electrons are produced in the tube.
(ii) State and explain the function of the grid.
(iii) State what would be observed on the screen if an a.c voltage is connected across the y-plates.
Date posted:
June 5, 2017
.
Answers (1)
-
Figure 15, shows two coils A and B placed close to each other. A is connected to a steady D.C. Supply and a switch, B is connected to a sensitive galvanometer.
(Solved)
Figure 15, shows two coils A and B placed close to each other. A is connected to a steady D.C. Supply and a switch, B is connected to a sensitive galvanometer.

(i) The switch is now closed. State the observations made on the galvanometer.
(ii) Explain what would be observed if the switch is then open.
Date posted:
June 5, 2017
.
Answers (1)
-
Figure 11 shows a test-tube whose cross-sectional area is 2 cm2 partially filled with lead shot floating vertically in water.
(Solved)
Figure 11 shows a test-tube whose cross-sectional area is 2 cm2 partially filled with lead shot floating vertically in water.

(i) Determine the: volume of the water displaced;
(ii) Determine weight of water displaced.
(iii) State the combined weight of the test-tube and the lead shot.
Date posted:
June 5, 2017
.
Answers (1)
-
Figure 13 shows a hydrometer with a thin stem floating in water in a beaker.
(Solved)
Figure 13 shows a hydrometer with a thin stem floating in water in a beaker.

State with a reason what is observed on the hydrometer when the temperature of the water is raised.
Date posted:
June 5, 2017
.
Answers (1)
-
Figure 13 shows a log of wood of mass 20 kg submerged in water in a pond and held in position by a string fixed to the bottom of the pond.
(Solved)
Figure 13 shows a log of wood of mass 20 kg submerged in water in a pond and held in position by a string fixed to the bottom of the pond.

Given that the density of water is 1000 kgm -3 and that of wood is 800 kgm -3, determine the;
(i) Volume of the log.
(ii) Upthrust of the log.
(iii) Tension in the string.
Date posted:
June 5, 2017
.
Answers (1)
-
The system in Figure below is in equilibrium.
(Solved)
The system in Figure below is in equilibrium.

When temperature of the water is raised the system is observed to tilt to the right. State the reason for this observation.
Date posted:
June 5, 2017
.
Answers (1)
-
Figure 14 shows a cork floating on water and held to the bottom of the beaker by a thin thread.
(Solved)
Figure 14 shows a cork floating on water and held to the bottom of the beaker by a thin thread.

(i) Name the forces acting on the cork
(ii) Describe how each of the forces mentioned in (i) above changes when water is added into the beaker until it fills up.
Date posted:
June 5, 2017
.
Answers (1)
-
Figure 13 shows a simple hydrometer.
(Solved)
Figure 13 shows a simple hydrometer.

(i) State the purpose of the lead shots in the glass bulb.
(ii) How would the hydrometer be made more sensitive?
(iii) Describe how the hydrometer is calibrated to measure relative density.
Date posted:
June 5, 2017
.
Answers (1)