73^0 C =73 + 273=346K
10^0 C=10 + 273 =283K
V1 =0.04m^3
=(V1/T1) =(V2/T2)
(0.04/346)=(V2/283)
V2=(0.04X283)/346
=0.03272m^3
Githiari answered the question on September 16, 2017 at 07:36
- Explain why convection currents in gases are set up much faster than convection currents in liquids. (Solved)
Explain why convection currents in gases are set up much faster than convection currents in liquids.
Date posted: September 16, 2017. Answers (1)
- In an experiment to estimate the size of a molecule of olive oil, a drop of oil of volume 0.24mm³ was placed on a clean...(Solved)
In an experiment to estimate the size of a molecule of olive oil, a drop of oil of volume 0.24mm³ was placed on a clean water surface. The oil spread into a patch of area 4.2 x 10⁴mm². Estimate the size of a molecule of olive oil and express your answer in meters.
Date posted: September 16, 2017. Answers (1)
- Explain how aquatic animals and plants survive in a lake whose top layer is ice at 0oC.(Solved)
Explain how aquatic animals and plants survive in a lake whose top layer is ice at 0oC.
Date posted: September 16, 2017. Answers (1)
- Explain why the smell of a rotting body is very strong when the sun is hot as compared to early in the morning.(Solved)
Explain why the smell of a rotting body is very strong when the sun is hot as compared to early in the morning.
Date posted: September 16, 2017. Answers (1)
- Explain how oiling still water controls the breeding of mosquitoes(Solved)
Explain how oiling still water controls the breeding of mosquitoes
Date posted: September 16, 2017. Answers (1)
- If the ball is spinning in the anticlockwise direction, the ball is seen to move upwards from its initial path. Explain(Solved)
If the ball is spinning in the anticlockwise direction, the ball is seen to move upwards from its initial path. Explain
Date posted: September 16, 2017. Answers (1)
- Distinguish between solid and liquid using molecular motion
(Solved)
Distinguish between solid and liquid using molecular motion
Date posted: September 16, 2017. Answers (1)
- Explain how the braking system of a car works.(Solved)
Explain how the braking system of a car works.
Date posted: September 15, 2017. Answers (1)
- A magnetized bar is passed over a bunsen flame several times. When tested it is found to be demagnetized. Explain this observation. (Solved)
A magnetized bar is passed over a bunsen flame several times. When tested it is found to be demagnetized. Explain this observation.
Date posted: September 15, 2017. Answers (1)
- An uncharged metal rod is brought close but not touching the cap of a charged electroscope causes a decrease in the divergence of the leaf.(Solved)
An uncharged metal rod is brought close but not touching the cap of a charged electroscope causes a decrease in the divergence of the leaf. Explain.
Date posted: September 15, 2017. Answers (1)
- Give a reason why an air bubble increases in volume as it ascends to the surface of the liquid in a boiler.(Solved)
Give a reason why an air bubble increases in volume as it ascends to the surface of the liquid in a boiler.
Date posted: September 15, 2017. Answers (1)
- Why are the caps of the cell of a lead acid accumulator opened when charging the battery?(Solved)
Why are the caps of the cell of a lead acid accumulator opened when charging the battery?
Date posted: September 12, 2017. Answers (1)
- Why do bus body builders build luggage compartments under the seats rather than on roof rack?(Solved)
Why do bus body builders build luggage compartments under the seats rather than on roof rack?
Date posted: September 9, 2017. Answers (1)
- I bought some red pens at sh 6 each and some blue pens at sh 4 each. If I bought 38 pens in total and paid sh 192 for them, find the number of red and blue pens I bought. (Solved)
I bought some red pens at sh 6 each and some blue pens at sh 4 each. If I bought 38 pens in total and paid sh 192 for them, find the number of red and blue pens I bought.
Date posted: June 8, 2017. Answers (1)
- Figure 8 shows a uniform light bar resting horizontally on corks floating on water in two beakers A and B. (Solved)
Figure 8 shows a uniform light bar resting horizontally on corks floating on water in two beakers A and B.
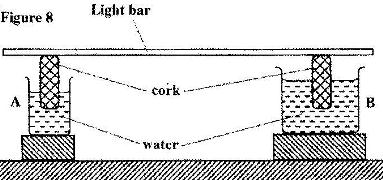
Explain why the bar tilts towards side A when equal amount of heat is supplied to each beaker.
Date posted: June 7, 2017. Answers (1)
- The light uniform bar in Figure 3 is in equilibrium. The two beakers A and B contain water at the same temperature. The two blocks are made of the same material. (Solved)
The light uniform bar in Figure 3 is in equilibrium. The two beakers A and B contain water at the same temperature. The two blocks are made of the same material.
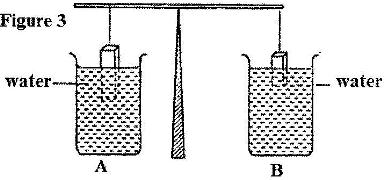
If the temperature of the water in beaker A is now raised, explain why the beam tips to side A. Assume the solid does not expand.
Date posted: June 7, 2017. Answers (1)
- Figure 11 shows a depletion layer in an unbiased p-n junction. (Solved)
Figure 11 shows a depletion layer in an unbiased p-n junction.
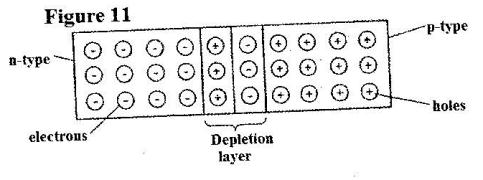
State how a battery can be used to make the depletion layer narrower.
Date posted: June 7, 2017. Answers (1)
- Figure 12 shows a depletion layer in an unbiased p-n junction. (Solved)
Figure 12 shows a depletion layer in an unbiased p-n junction.
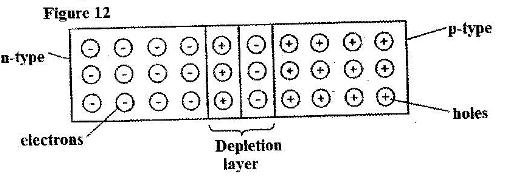
State how a battery can be used to make the depletion layer narrower.
Date posted: June 7, 2017. Answers (1)
- Figure 6 (a) and Figure 6 (b) show a p-n junction connected to a battery. It is observed that the current in figure (6) (a) is greater than the current in figure 6 (b). (Solved)
Figure 6 (a) and Figure 6 (b) show a p-n junction connected to a battery. It is observed that the current in figure (6) (a) is greater than the current in figure 6 (b).
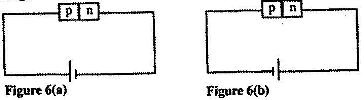
State the reason for this observation.
Date posted: June 7, 2017. Answers (1)
- Figure 5 shows the circuit of a rectifier using four diodes D1, D2, D3 and D4. (Solved)
Figure 5 shows the circuit of a rectifier using four diodes D1, D2, D3 and D4.
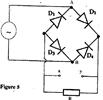
Explain how a rectified output is produced from the set up when an a.c input is connected across AB.
Date posted: June 6, 2017. Answers (1)