a)CO(g) + PbO (s)------------> Pb (s) + CO2 (g)
b)Extraction of metals; through reduction of the metal oxide
c)Hydrogen
Ammonia
Githiari answered the question on September 18, 2017 at 06:41
-
a)Why is the percentage of carbon(IV)oxide in the atmosphere fairly constant? (b)Calculate the volume of carbon(IV)oxide in 8,000 m3 of air contained in a hall.
(Solved)
a)Why is the percentage of carbon(IV)oxide in the atmosphere fairly constant?
b)Calculate the volume of carbon(IV)oxide in 8,000 m3 of air contained in a
Date posted:
September 18, 2017
.
Answers (1)
-
Which two elements will react more vigorously with each other between fluorine, neon, sodium, magnesium?
(Solved)
Which two elements will react more vigorously with each other between fluorine, neon, sodium, magnesium?
Date posted:
September 17, 2017
.
Answers (1)
-
a)Given that the mass of copper obtained in an o extraction was 210kg, determine the percentage purity of the ore used (copper pyrites) if 810kg...
(Solved)
a)Given that the mass of copper obtained in an o extraction was 210kg, determine the percentage purity of the ore used (copper pyrites) if 810kg of it was fed to the 1st roasting furnace. (Cu = 63.5, Fe = 56, S = 32.0)
b)Give 2 effects that the process above could have on the environment.
Date posted:
September 14, 2017
.
Answers (1)
-
a)Using ionic equation explain how sodium carbonate can be used to soften hard water.(b)Other than softening of hard water give 2 other uses of sodium...
(Solved)
a)Using ionic equation explain how sodium carbonate can be used to soften hard water.
b)Other than softening of hard water give 2 other uses of sodium carbonate.
Date posted:
September 14, 2017
.
Answers (1)
-
Predict the pH of the oxide of sulphur in water.
(Solved)
Predict the pH of the oxide of sulphur in water.
Date posted:
September 14, 2017
.
Answers (1)
-
Explain why sulphur has a higher boiling point compared to that of oxygen.
(Solved)
Explain why sulphur has a higher boiling point compared to that of oxygen.
Date posted:
September 14, 2017
.
Answers (1)
-
A mixture consists of sulphur powder and iron filings.(i) Describe how to obtain sulphur from the mixture using methylbenzene.
(Solved)
(i) Describe how to obtain sulphur from the mixture using methylbenzene. (3 marks)
(ii) Is the mixture homogeneous or heterogeneous? Explain. (2 marks)
Date posted:
September 13, 2017
.
Answers (1)
-
What is the importance of the shape of a conical flask?
(Solved)
What is the importance of the shape of a conical flask?
Date posted:
September 13, 2017
.
Answers (1)
-
Using dot and cross to represent electrons draw a diagram to illustrate bonding in the sulphide of a substance with a pH of 2.2
(Solved)
Using dot and cross to represent electrons draw a diagram to illustrate bonding in the sulphide of a substance with a pH of 2.2
Date posted:
September 10, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
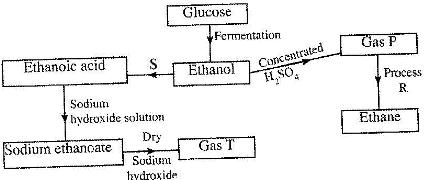
(i) State the conditions necessary for fermentation of glucose to take place.
(ii) State the reagent that can be used to carry out process S.
(iii) Identify gases P and T.
Date posted:
June 7, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follows.
(Solved)
Study the flow chart below and answer the questions that follows.
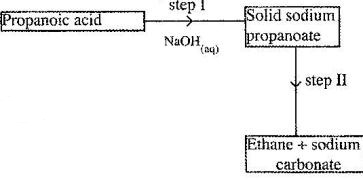
(a) Name the process in step I.
(b) Identify the reagent in step II.
(c) Give one use of ethane.
Date posted:
June 7, 2017
.
Answers (1)
-
The table below shows the relative molecular masses and boiling points of pentane and ethanoic acid.
(Solved)
The table below shows the relative molecular masses and boiling points of pentane and ethanoic acid.
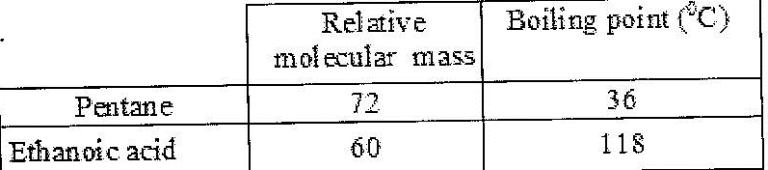
Explain the large difference in boiling point between ethanoic acid and pentane.
Date posted:
June 7, 2017
.
Answers (1)
-
The structure below represents a type of a cleansing agent.
(Solved)
The structure below represents a type of a cleansing agent.
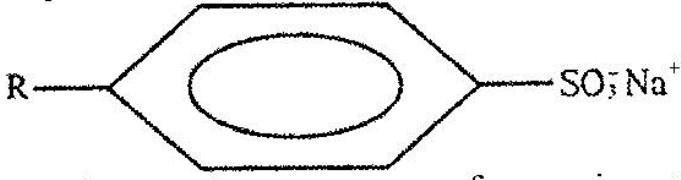
Describe how the cleansing agent removes grease from a piece of cloth.
Date posted:
June 7, 2017
.
Answers (1)
-
Study the flow chart below and use it to answer the questions that follow:
(Solved)
Study the flow chart below and use it to answer the questions that follow:
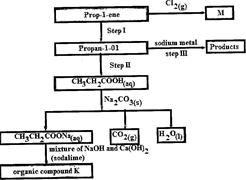
(i) Identify the organic compound K.
(ii) Write the formula of M
(iii) Give one reagent that can be used in:
(I) Step I; (II) step II
Date posted:
June 7, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
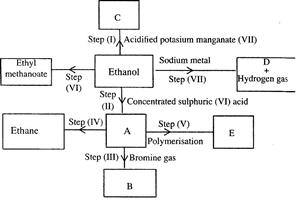
(i) (I) What is observation will be made in step I?
(II) Describe a chemical test that can be carried out to show the identity of compound C.
(ii) Give the names of the following: I. E II. Substance D.
Date posted:
June 7, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
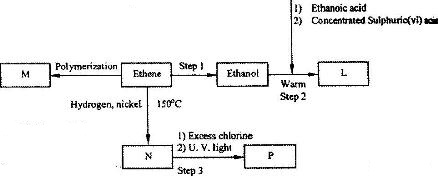
(i) Name the compounds:
(I) L
(II) N
(ii) Give the reagent and the condition used in step 1.
(iii) State the type of reaction that takes place in:
(I) Step 2; (II) step 3;
Date posted:
June 7, 2017
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
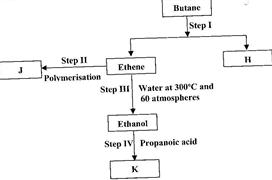
(i) State the conditions for the reaction in Step I to occur.
(ii) Identify substance J.
(iii) State one disadvantage of the continued use of substance such as J.
Date posted:
June 7, 2017
.
Answers (1)
-
Ethanol obtained from glucose can be converted to ethene as shown below;
(Solved)
Ethanol obtained from glucose can be converted to ethene as shown below;
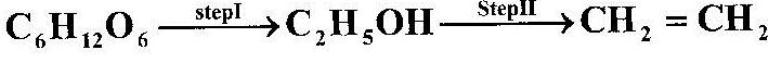
Name and describe the process that takes place in steps I and II.
Date posted:
June 7, 2017
.
Answers (1)
-
Propane can be changed into methane and ethane as shown in the equation below.
(Solved)
Propane can be changed into methane and ethane as shown in the equation below.

Name the process undergone by propane
Date posted:
May 25, 2017
.
Answers (1)
-
The flow chart below shows a series of reactions starting with ethanol. Study it and answer the questions that follow.
(Solved)
The flow chart below shows a series of reactions starting with ethanol. Study it and answer the questions that follow.

(i) Name: I. Process A. II. Substances B and C
(ii) Explain why it is necessary to use high pressure to change gas B into the polymer.
Date posted:
May 25, 2017
.
Answers (1)