(i)Heat lost=mLv+mc(100-T)
H=(0.003×2260000)+
(0.003×4200[100-T])
H=6780+1260-12.6T
(ii)Heat gained=Mc(T-10)
H=0.4×4200(T-10)
H=1680T-16800
(iii) 1680T-16800=6780+1260-12.6T
1692.6T=24840
T= 14.68°C
jacktonenzoya answered the question on August 29, 2019 at 06:53
- The graph shows variation of extension and stretching force F for a spring which obeys Hooke’s law.
(i) Determine the spring constant in SI units .
(ii)...(Solved)
The graph shows variation of extension and stretching force F for a spring which obeys Hooke’s law.
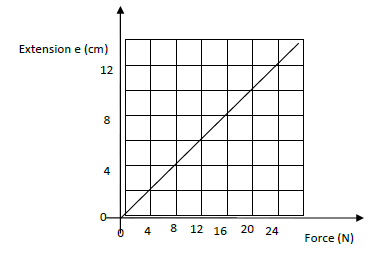
(i) Determine the spring constant in SI units .
(ii) The energy stored when the extension is 20 cm.
Date posted: August 6, 2019. Answers (1)
- The figure below shows two identical metallic cans A and B, filled with water at room temperature. An electric heater is placed at equal distances...(Solved)
The figure below shows two identical metallic cans A and B, filled with water at room temperature. An electric heater is placed at equal distances from A and B.

In the figure below sketch a graph to show the variation of temperature with time for the two surfaces.

Date posted: August 6, 2019. Answers (1)
- A ball bearing of mass 1.5 x 10-4 kg is held between the anvil and spindle of a micrometer screw
gauge as shown in the figure...(Solved)
A ball bearing of mass 1.5 x 10-4 kg is held between the anvil and spindle of a micrometer screw
gauge as shown in the figure below.
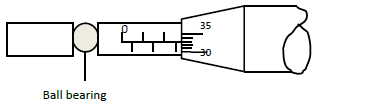
Before the instrument was the zero error was found to be 0.03 mm.
(i) What is the diameter of the ball bearing?
(ii) Find the density of the ball bearing correct to 3 significant figures and in SI units.
Date posted: August 6, 2019. Answers (1)
- Explain why when graduating the upper fixed point of a thermometer; the bulb is put in steam
above boiling water and not boiling water.(Solved)
Explain why when graduating the upper fixed point of a thermometer; the bulb is put in steam
above boiling water and not boiling water.
Date posted: August 6, 2019. Answers (1)
- The figure below shows a soap film formed on a metal ring and a loop of thread inside it.
(i) Explain what will happen when the...(Solved)
The figure below shows a soap film formed on a metal ring and a loop of thread inside it.

(i) Explain what will happen when the film is punctured by a needle at X.
(ii) What is the name given to the force acting on the thread?
Date posted: August 6, 2019. Answers (1)
- In an experiment to determine the relative density of a substance using a density bottle the
following measurements were taken.
- Mass of empty density bottle =...(Solved)
In an experiment to determine the relative density of a substance using a density bottle the
following measurements were taken.
- Mass of empty density bottle = 43.2 g
- Mass of bottle full of water = 66.4 g
- Mass of bottle filled with liquid X = 68.2g
Use the data to determine the density of the liquids.
Date posted: August 6, 2019. Answers (1)
- The figure below shows the path of ray of yellow light through a glass prism. The speed of
yellow light in the prism is 1.8 x...(Solved)
The figure below shows the path of ray of yellow light through a glass prism. The speed of
yellow light in the prism is 1.8 x 108m/s.

(i) Determine the refractive index of the prism material (Speed of light in vacuum, C = 3.0 x 108m/s)
(ii) Show on the same diagram, the critical angle C and hence determine its value.
Date posted: August 6, 2019. Answers (1)
- The diagram below shows a narrow beam of white light onto a glass prism.
(i) What is the name of the phenomenon represented in the diagram?...(Solved)
The diagram below shows a narrow beam of white light onto a glass prism.
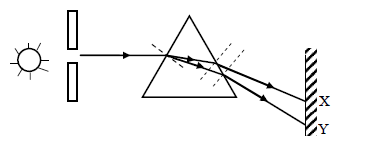
(i) What is the name of the phenomenon represented in the diagram?
(ii) Name the color at X and Y
Date posted: August 6, 2019. Answers (1)
- A 5µF capacitor is charged to a potential difference of 200V and isolated. It is then connected
to a 10µF capacitor.Find
(i) The resultant potential difference across...(Solved)
A 5μF capacitor is charged to a potential difference of 200V and isolated. It is then connected
to a 10μF capacitor.Find
(i) The resultant potential difference across the combination
(ii) Energy stored before connection
(iii) Total energy in the capacitors after connection
Date posted: August 6, 2019. Answers (1)
- The figure shows a human eye with a defect
(i) Identify the defect
(ii) Explain how the defect could be corrected
(iii) Draw a suitable diagram...(Solved)
The figure shows a human eye with a defect

(i) Identify the defect
(ii) Explain how the defect could be corrected
(iii) Draw a suitable diagram to show the correction of the defect
Date posted: August 6, 2019. Answers (1)
- When the switch S is kept open in the circuit shown below the voltmeter reads 1.5V. When the switch is closed, the readings drops to...(Solved)
When the switch S is kept open in the circuit shown below the voltmeter reads 1.5V. When the switch is closed, the readings drops to 1.3V and the current through the resistor is 0.5A.

(i) What is the e.m.f of the cell?
(ii) What the terminal voltage of the cell?
(iii) Calculate the value of R.
Date posted: August 6, 2019. Answers (1)
- The figure below shows how a student set up a circuit using 3 identical bulbs X, Y and Z
each rated “12V, 2.0A”
(i) When operating normally,...(Solved)
The figure below shows how a student set up a circuit using 3 identical bulbs X, Y and Z
each rated “12V, 2.0A”
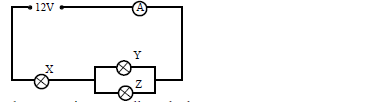
(i) When operating normally, calculate the resistance of one of the bulbs.
(ii) Calculate the effective resistance of the three bulbs.
(iii) What will be reading of the ammeter?
(iv) Draw a circuit diagram showing the three bulbs connected in such a way that they would all work at the same brightness especially if they are not identical.
Date posted: August 6, 2019. Answers (1)
- State two quantities that are used to determine whether accumulator require recharging or not.(Solved)
State two quantities that are used to determine whether accumulator require recharging or not.
Date posted: August 6, 2019. Answers (1)
- State one advantage of using an optical fibre in communication.(Solved)
State one advantage of using an optical fibre in communication.
Date posted: August 6, 2019. Answers (1)
- The figure below shows how the displacement varies for a certain wave.
Determine the frequency of the wave.(Solved)
The figure below shows how the displacement varies for a certain wave.

Determine the frequency of the wave.
Date posted: August 6, 2019. Answers (1)
- The figure below shows part of the electromagnetic spectrum.
(a) Identify radiation W.
(b) State one the use of radiation W above.(Solved)
The figure below shows part of the electromagnetic spectrum.

(a) Identify radiation W.
(b) State one the use of radiation W above.
Date posted: August 6, 2019. Answers (1)
- Explain why soft iron keepers are suitable for storing magnets.(Solved)
Explain why soft iron keepers are suitable for storing magnets.
Date posted: August 6, 2019. Answers (1)
- The figure below shows a ray of light incident on a plane mirror.(Solved)
The figure below shows a ray of light incident on a plane mirror.
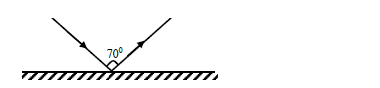
The plane mirror is then rotated clockwise through an angle of 200 keeping the incident ray
fixed. Determine the new angle of reflection.
Date posted: August 6, 2019. Answers (1)
- Explain why it is easier to loosen a tight nut using a spanner with long handle(Solved)
Explain why it is easier to loosen a tight nut using a spanner with long handle
Date posted: June 8, 2019. Answers (1)
- State four significance of Karst regions.(Solved)
State four significance of Karst regions.
Date posted: June 4, 2019. Answers (1)